- MSD expands domestic clinical trial cooperation
- by Whang, byung-woo | translator Alice Kang | May 20, 2025 06:00am
With open innovation playing an increasingly important role in new drug research and development (R&D), MSD is expanding its ties with Korea, which plays a pivotal role in its global clinical trials.
On the 19th, MSD Korea held R:IM (Notification) DAY and highlighted changes in R&D trends under the theme of “A New Paradigm in the Pharmaceutical Industry: Global Clinical Trends and MSD's Vision.”
Korea plays a pivotal role in global MSD cancer research
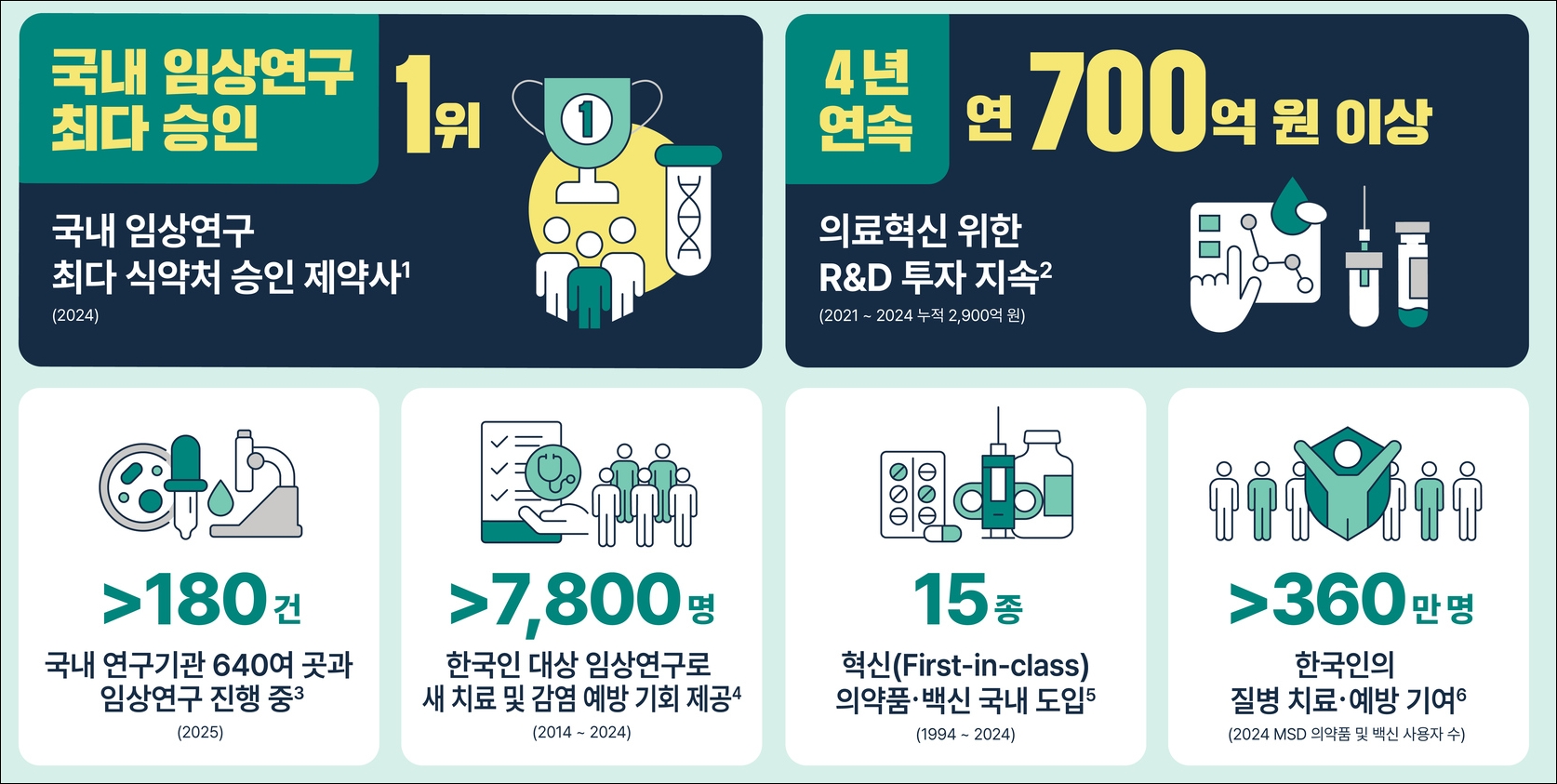
The reason why MSD Korea's performance in the domestic R&D field is attracting attention is that it received the most clinical trial approvals last year.
In 2024, MSD Korea received 36 clinical trial approvals from the Ministry of Food and Drug Safety, the most among domestic pharmaceutical companies.
During the same period, AstraZeneca Korea (22), AD pharma (19), AbbVie Korea (17), and Boehringer Ingelheim Korea (15) received clinical trial approvals from the MFDS.
In particular, MSD Korea has been leading Korea’s medical ecosystem by investing more than KRW 70 billion for 4 consecutive years since 2021, with a cumulative total of KRW 290 billion in research and development costs. This proves the company’s R&D competitiveness in terms of the number of clinical trials, costs, as well as its quality and quantity.

Hyunjoo Lee, Executive Director of Clinical Research at MSD Korea, who made a presentation on that day, said, “We are focusing on research and development of new drugs that have been proven to be effective and safe for Koreans in cooperation with domestic research institutes and academic societies.”
MSD Korea has also been playing a central role in MSD's global anticancer drug clinical trials.
Currently, the company is conducting over 180 clinical trials in collaboration with more than 640 domestic research institutions, with the largest portion (161 studies) focused on oncology.
Although Korean institutions account for only 3% (518) of the 14,770 global institutions conducting anticancer drug clinical trials, these institutions are responsible for 73% of MSD's global cancer drug trials, demonstrating the concentrated utilization of Korea's research capabilities.
Major hospitals in Korea, such as Seoul National University Hospital, Samsung Medical Center, Asan Medical Center, and Severance Hospital, are among the top institutions leading MSD's global clinical trials, which is considered a testament to Korea's internationally recognized clinical research capabilities.
In addition, Korea ranks fourth in the world in terms of the number of patients enrolled in MSD's global anticancer drug clinical trials, following the United States, China, and Japan.
Accelerating digitalization... challenge remains on establishing a sustainable cooperation model
Another trend in the global pharmaceutical industry's clinical trials is the integration of digital technology.
MSD is also actively promoting the introduction of digital technology and artificial intelligence (AI) to keep pace with this trend.
A representative example is the increased use of innovative clinical designs such as umbrella, basket, and adaptive protocols in clinical trial design.
“MSD is systematically introducing AI and machine learning technologies into the drug discovery and development process to accelerate pipeline diversification,” said Lee. ”MSD has developed AI tools that enable more accurate evaluation of the safety and efficacy of the active substances in its pipeline during the preclinical development stage.”
In the long term, MSD Korea aims to become a trusted R&D partner, a bridgehead for domestic pharmaceutical and biotech companies to gain global research experience and enter the market, and foster an ecosystem for innovative new drug development.
This includes improving the regulatory environment in Korea, expanding clinical trial infrastructure, and building more active partnerships with domestic research institutions and biotech companies.
Currently, MSD Korea is expanding its partnership by conducting joint clinical trials with major domestic biotech companies for the combination therapy of the immuno-oncology drug Keytruda.
In the future, the company plans to expand this collaboration to various other fields to build a sustainable ecosystem that will enhance the global competitiveness of the Korean pharmaceutical industry.
Lee stated, “To enhance the global competitiveness of Korea's pharmaceutical and biotechnology industry, it is essential for the government, academia, and industry to collaborate closely. We will strive to establish a sustainable collaboration model to position Korea as a global hub for pharmaceutical research.”
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.
- [Op-Ed] Patients, no time left for 'new drug comb therapies'
- Special Contribution | Eo, Yun-Ho









