- Policy
- Comb cancer therapy issue has been resolved
- by Whang, byung-woo Apr 21, 2025 05:53am
- Changes will be brought to the health system as a patient's existing co-payment amount will remain the same for ongoing chemotherapy when a reimbursed cancer drug combined with a newly developed, non-reimbursed new drug. Previously, insurance coverage for combination therapy comprising two drugs was unavailable, placing a financial burden on
- Policy
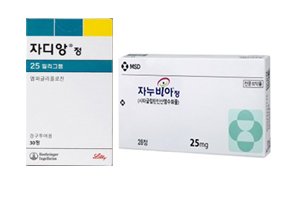
- CKD approved for Januvia+Jardiance+metformin combo
- by Lee, Hye-Kyung Apr 18, 2025 05:59am
- Chong Kun Dang, which launched a combination drug that combines the DPP-4 inhibitor 'Januvia (sitagliptin)' and the SGLT-2 inhibitor 'Jardiance (empagliflozin),' has received approval for a triple combination drug that adds metformin to the same combination just one week later. On the 16th, the Ministry of Food and Drug Safety approved
- Policy
- Original-generic collusion prevention law introduced
- by Lee, Jeong-Hwan Apr 18, 2025 05:59am
- A bill has been proposed in the National Assembly to prevent collusion between original drug manufacturers and generic drug manufacturers from maintaining the domestic sales status of original drugs through illegal means. On the 17th, Representative Young-Seok Seo (Gyeonggi Province, Bucheon City, National Assembly Health and Welfare Commi
- Policy
- Co-payments retained for non-reimb drug+reimb drug comb
- by Lee, Jeong-Hwan Apr 18, 2025 05:57am
- The Ministry of Health and Welfare (MOHW) has decided that, even if an additional or combination anticancer drug is added to a chemotherapy regimen already covered by health insurance, the initial chemotherapy will continue to be subject to the patient’s existing co-payment. Boehringer Ingelheim’s idiopathic pulmonary fibrosis and fibro
- Policy
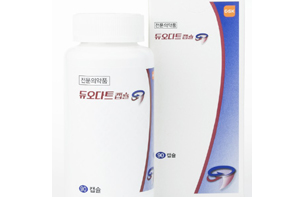
- Will a 'Duodart' generic for prostatate drug be available?
- by Lee, Hye-Kyung Apr 16, 2025 05:55am
- A generic version of GSK's benign prostatic hyperplasia (BPH) combination product 'Duodart Capsule (dutasteride·tamsulosin hydrochloride),' which drove the success of the combination drug market for urology in Korea, is now under development. On the 14th, the Ministry of Food and Drug Safety (MFDS) approved an open-label, randomized, two
- Policy
- Global pharma proposes 'indication-based pricing system'
- by Lee, Jeong-Hwan Apr 16, 2025 05:55am
- Overseas global pharmaceutical companies with subsidiaries in Korea are currently voicing the adoption of policies aimed at resolving 'reimbursement inequities' for therapies for severe and rare diseases and implementing an 'indication-based pricing system' amid an early presidential election. While the Korean government maintains that an
- Policy
- DPK’s election pledge includes substitution dispensing
- by Lee, Jeong-Hwan Apr 15, 2025 05:54am
- The Democratic Party of Korea will include a policy to support domestically developed new drugs through a drug pricing system that links the investment rate (amount) of research and development (R&D) by pharmaceutical companies with a drug’s insurance price ceiling and drug price reduction rate in its presidential election pledge. DPK’s
- Policy
- NHIS prepares measures to implement dual drug pricing system
- by Lee, Tak-Sun Apr 15, 2025 05:54am
- The National Health Insurance Service (NHIS) is preparing a procedure for implementing the dual drug pricing system for domestically developed new drugs and will collect industry opinion this month. The measure seeks to prepare guidelines for actual negotiations, as the MOHW's notification last month included a provision for the dual d
- Policy
- NHIS opens a non-reimbursement information portal
- by Lee, Tak-Sun Apr 11, 2025 06:01am
- The National Health Insurance Service (NHIS) announced that it will launch a 'non-reimbursement information portal' on the 10th to help people easily understand information on non-reimbursed items at a glance to improve their right to know and promote rational medical use. Unlike reimbursed items, where prices and standards of care are se
- Policy
- Cost of cell&gene therapies for clinical patients set
- by Lee, Jeong-Hwan Apr 10, 2025 05:56am
- The government has set out to create standards for the 'cost of clinical trial drugs' to be used as an indicator when giving patients expensive cell and gene therapy drugs at the clinical trial stage. This is to rationally calculate the cost that patients must pay when they are administered investigational drugs for therapeutic purposes.