- Policy
- Public-private meetings on improving drug pricing system
- by Lee, Tak-Sun Mar 20, 2025 06:00am
- Attention has been gathered to recent public-private meetings regarding improving drug pricing system. This month, a public-private discussion session hosted by the Ministry of Health and Welfare (MOHW) and a meeting to discuss the negotiation system for expanded use of scope were held. On March 26, a regular meeting session was attende
- Policy
- Drug briefing on Enhertu's NSCLC reimb has been requested
- by Lee, Tak-Sun Mar 19, 2025 06:02am
- It has been reported that Daiichi Sankyo, which is aiming for Enhertu (trastuzumab deruxtecan)'s expanded reimbursement for HER2-mutant non-small cell lung cancer (NSCLC), will hold a drug briefing session with the Health Insurance Review & Assessment Service (HIRA). In April 2024, Enhertu was added to the reimbursement listing in South K
- Policy
- Five drugs all pass DREC's reimb review in February
- by Lee, Tak-Sun Mar 17, 2025 05:59am
- All five products, including new drugs and drugs that sought expanded indications, which were reviewed by the Health Insurance Review and Assessment Service's Drug Reimbursement Evaluation Committee in February, have passed review and will be negotiating the drug’s price with the National Health Insurance Service. Drugs that are subject
- Policy
- Mandatory reporting of market supply suspended drugs
- by Lee, Hye-Kyung Mar 13, 2025 05:58am
- The mandatory reporting is being considered to include drug products of suspended production·importation and supply, and 'supply shortage drugs' of which supply has been halted for more than 1 month. The Ministry of Food and Drug Safety (MFDS; Minister, Yu-Kyoung Oh) stated that it would announce on March 11 an administrative notice of revis
- Policy
- Shortage of Synagis supply announced…normalized by May
- by Lee, Hye-Kyung Mar 13, 2025 05:57am
- With a shortage in the supply of Synagis (palivizumab), a preventive antibody for respiratory syncytial virus (RSV), which is a main cause of pneumonia and bronchiolitis in infants and young children, expected in Korea, concern arises on how the lack of supply may hinder disease prevention for children in high-risk groups. On the 11th, As
- Policy
- New types of risk-sharing agreements added for reimbursement
- by Lee, Tak-Sun Mar 12, 2025 05:57am
- The Health Insurance Review and Assessment Service has established two types of risk-sharing schemes, including initial treatment cost reimbursement (Fixed cost refund at initial treatment) and outcome-based reimbursement, to the detailed criteria for drugs subject to negotiation. This appears to reflect the additional content included in
- Policy
- Tadalafil, only concern for abuse in erectile dysfunction
- by Lee, Hye-Kyung Mar 11, 2025 05:54am
- ’Tadalafil,’ whose indications have been expanded to treat not only erectile dysfunction but also benign prostatic hyperplasia, is expected to partially get rid of the shackles of being a drug with concerns for misuse and abuse. According to the Ministry of Food and Drug Safety's “Partial Amendment to the Regulations on Designation of D
- Policy
- Keytruda will be considered for DREC next time
- by Lee, Tak-Sun Mar 11, 2025 05:54am
- The expanded use of scope application of the immune cancer drug Keytruda, which passed the Cancer Disease Review Committee, is expected to be considered for the Drug Reimbursement Evaluation Committee (DREC) review as soon as April. Previously, the 2nd DREC review for 2025, held on March 6, did not include Keytruda. As Keytruda's DREC rev
- Policy
- 734 SGLT2is add precautions for ketoacidosis
- by Lee, Hye-Kyung Mar 10, 2025 05:50am
- Precautions for antidiabetic SGLT2 inhibitors will be strengthened in the near future. 734 ertugliflozin, empagliflozin, and dapagliflozin drugs that have been approved in Korea are expected to be subject to the changed regulations. The Ministry of Food and Drug Safety will prepare an ‘Order for labeling changes (draft)’ based on the
- Policy
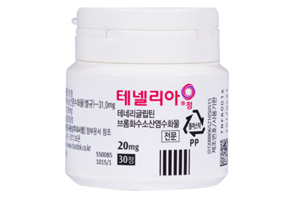
- Handok expands its Tenelia combo lineup
- by Lee, Hye-Kyung Mar 7, 2025 05:56am
- Handok is speeding up the development of a combination drug that combines DPP-4 inhibitor diabetes treatment Tenelia (teneligliptin hydrobromide hydrate)' with SGLT-2 inhibitor 'Jadiance (Empagliflozin). On the 4th, the Ministry of Food and Drug Safety approved an open-label, randomized, empty stomach, single orally administered, two-arm,