- Policy
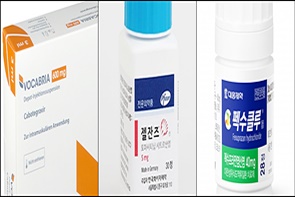
- Vocabria·Rekambys newly listed…expanded reimb for Xeljanz
- by Lee, Jeong-Hwan Mar 26, 2025 06:00am
- The HIV treatments Vocabria (cabotegravir) and Rekambys (rilpivirine) will be newly added to the National Health Insurance reimbursement list next month. The scope of reimbursement will be expanded for Pfizer's autoimmune diseases treatment Xeljanz (tofacitinib), Novartis' Cosentyx (secukinumab), and Daewoong's erosive gastroesophageal ref
- Policy
- New law proposed for the cancer and rare disease fund
- by Lee, Jeong-Hwan Mar 25, 2025 05:54am
- A bill to establish a new fund for cancer and rare diseases to strengthen patient access to ultra-high-priced drugs has been proposed to the National Assembly. The fund will be raised through transfers and deposits from other funds, such as the lottery fund. On the 24th, National Assembly member Myeong-ok Seo (People Power Party), a me
- Policy
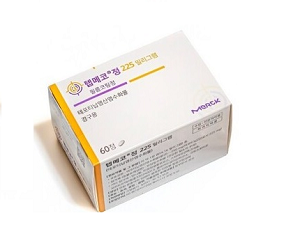
- Tepmetko, Tevimbra granted reimbursement in Korea
- by Lee, Tak-Sun Mar 24, 2025 05:52am
- New anticancer drugs Tepmetko and Tevimbra will be included in the list of reimbursed drugs as of April 1 in Korea. In addition, the economic burden on the patients is expected to be significantly reduced as the co-insurance rate for abiraterone acetate drugs such as Zytiga has been reduced for the first-line treatment of castration-resis
- Policy
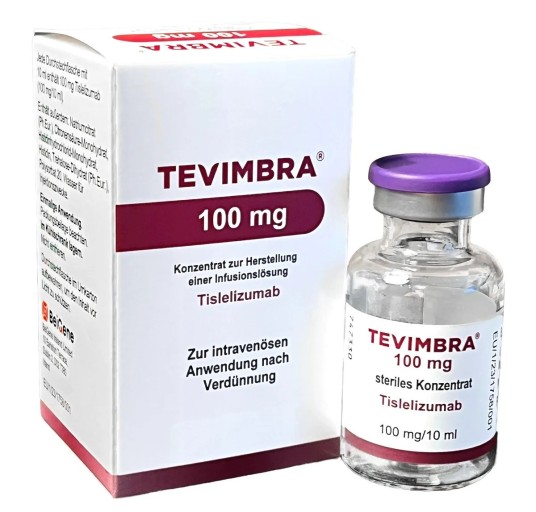
- How Tevimbra was reimbursed in Korea first
- by Lee, Tak-Sun Mar 24, 2025 05:52am
- BeiGene Korea's Tevimbra will be reimbursed in Korea from next month as a second-line treatment for esophageal squamous cell carcinoma, a type of esophageal cancer. It is the first immuno-oncology drug to be covered for esophageal cancer. In particular, the drug is drawing attention as it was covered in South Korea before the A8 countries
- Policy
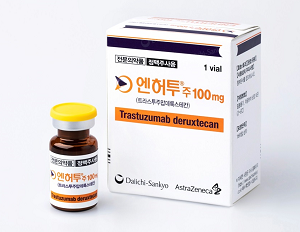
- Enhertu’s reimb may be extended to gastric cancer
- by Lee, Tak-Sun Mar 21, 2025 05:59am
- The number of patients who can use the new antibody-drug conjugate (ADC) anticancer drug ‘Enhertu Inj (trastuzumab deruxtecan, Daiichi Sankyo Korea), which has been reimbursed since April last year, is expected to be expanded to cover eligible gastric cancer patients. This is an expansion in the scope of existing patients eligible for reimb
- Policy
- Public-private meetings on improving drug pricing system
- by Lee, Tak-Sun Mar 20, 2025 06:00am
- Attention has been gathered to recent public-private meetings regarding improving drug pricing system. This month, a public-private discussion session hosted by the Ministry of Health and Welfare (MOHW) and a meeting to discuss the negotiation system for expanded use of scope were held. On March 26, a regular meeting session was attende
- Policy
- Drug briefing on Enhertu's NSCLC reimb has been requested
- by Lee, Tak-Sun Mar 19, 2025 06:02am
- It has been reported that Daiichi Sankyo, which is aiming for Enhertu (trastuzumab deruxtecan)'s expanded reimbursement for HER2-mutant non-small cell lung cancer (NSCLC), will hold a drug briefing session with the Health Insurance Review & Assessment Service (HIRA). In April 2024, Enhertu was added to the reimbursement listing in South K
- Policy
- Five drugs all pass DREC's reimb review in February
- by Lee, Tak-Sun Mar 17, 2025 05:59am
- All five products, including new drugs and drugs that sought expanded indications, which were reviewed by the Health Insurance Review and Assessment Service's Drug Reimbursement Evaluation Committee in February, have passed review and will be negotiating the drug’s price with the National Health Insurance Service. Drugs that are subject
- Policy
- Mandatory reporting of market supply suspended drugs
- by Lee, Hye-Kyung Mar 13, 2025 05:58am
- The mandatory reporting is being considered to include drug products of suspended production·importation and supply, and 'supply shortage drugs' of which supply has been halted for more than 1 month. The Ministry of Food and Drug Safety (MFDS; Minister, Yu-Kyoung Oh) stated that it would announce on March 11 an administrative notice of revis
- Policy
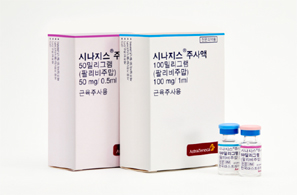
- Shortage of Synagis supply announced…normalized by May
- by Lee, Hye-Kyung Mar 13, 2025 05:57am
- With a shortage in the supply of Synagis (palivizumab), a preventive antibody for respiratory syncytial virus (RSV), which is a main cause of pneumonia and bronchiolitis in infants and young children, expected in Korea, concern arises on how the lack of supply may hinder disease prevention for children in high-risk groups. On the 11th, As