- Company
- Fruzaqla may be prescribed at general hospitals in KOR
- by Eo, Yun-Ho Sep 4, 2025 06:12am
- ] The new colon cancer drug Fruzaqla is may now be prescribed at general hospitals in Korea. According to industry sources, Fruzaqla (fruquintinib), a colorectal cancer therapy from Takeda Korea that selectively inhibits vascular endothelial growth factor receptors (VEGFR)-1, 2, and 3, has passed the drug committees (DC) of 41 major medica
- Company
- Novo Nordisk, Kakao Health sign digital healthcare MOU
- by Cha, Jihyun Sep 4, 2025 06:09am
- Kakao Healthcare (CEO Hee Hwang) announced on the 3rd that it has signed a memorandum of understanding with the multinational pharmaceutical company, Novo Nordisk Korea (General Manager Kasper Roseeuw Poulsen) to build a digital healthcare ecosystem for obesity and diabetes patients. The signing ceremony, held on September 2 at Novo Nordisk K
- Company
- Moderna’s latest variant-targeted COVID-19 vaccine approved
- by Whang, byung-woo Sep 3, 2025 06:08am
- Moderna Korea announced on the 1st that its LP.8.1 variant-targeted COVID-19 vaccine, ‘Spikevax LP Inj’, has been approved by the Ministry of Food and Drug Safety (MFDS). Spikevax LP has been confirmed to induce broad cross-immune responses against currently circulating variants, including the LP.8.1 strain, and it is authorized for use in
- Company
- 'Prevenar 20' now available at general hospitals
- by Eo, Yun-Ho Sep 2, 2025 06:11am
- The pneumococcal conjugate vaccine 'Prevenar 20,' which will soon be included in the National Immunization Program (NIP), is becoming available for prescription at general hospitals. According to industry sources, Pfizer Korea's Prevenar 20 has passed the drug committees (DC) of tertiary general hospitals, including Samsung Medical Cente
- Company
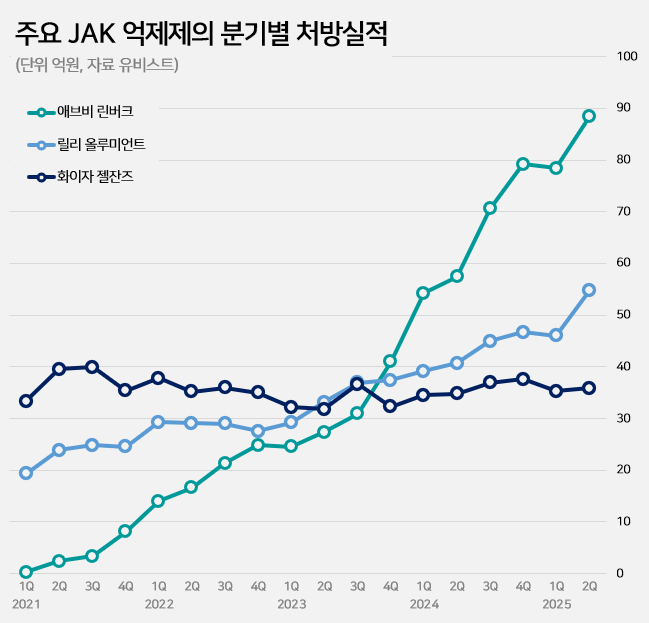
- Patent suits active to release Rinvoq generics
- by Kim, Jin-Gu Sep 2, 2025 06:10am
- The race to launch generics of AbbVie’s Rinvoq (upadacitinib), an oral treatment for autoimmune diseases, is showing signs of intensifying patent disputes. With the JAK inhibitor market expanding rapidly and Rinvoq solidifying its dominance, multiple companies are expected to mount patent challenges. According to industry sources on the
- Company
- China's presence rises amid early-stage ADC candidate deals
- by Son, Hyung Min Sep 2, 2025 06:10am
- With competition in the antibody-drug conjugate (ADC) market intensifying, global deal trends are undergoing a clear shift. Transactions are now more active in the preclinical and early clinical stages, rather than in late-stage candidates, with collaborations between global pharma and Chinese companies standing out. Since the success of Enhertu
- Company
- How will fate fare for Roche’s two anticancer drugs?
- by Eo, Yun-Ho Sep 1, 2025 06:05am
- The industry’s eyes are on whether Roche's two anti-cancer drugs, which have faced repeated failures, will succeed in securing reimbursement listing this time. According to industry sources, Roche Korea's treatment for refractory diffuse large B-cell lymphoma(DLBCL), ‘Polivy (polatuzumab vedotin)’ and the PD-L1 inhibitor-based immunoth
- Company
- COVID-19 response more important for high-risk groups
- by Whang, byung-woo Sep 1, 2025 06:04am
- While COVID-19 has entered an endemic phase and become a resident disease in daily life, it remains a significant threat to high-risk groups like the elderly. In fact, the outbreak of COVID-19, which had slowed down for a while, has been showing an upward trend again. According to the Korea Disease Control and Prevention Agency's Infectious D
- Company
- Capvaxive release imminent in the pneumococcal vaccine mkt
- by Whang, byung-woo Sep 1, 2025 06:03am
- MSD's adult-specific 21-valent protein-conjugated vaccine Capvaxive was granted marketing authorization in Korea by the Ministry of Food and Drug Safety and has emerged as a new competitor in the domestic adult pneumococcal vaccine market. With Prevenar 20 now launched in the market previously contested by Prevenar 13 and Vaxneuvance, Capvaxi
- Company
- CKD says, "New ADC drug enters first clinical trials"
- by Son, Hyung Min Sep 1, 2025 06:03am
- Chong Kun Dang is accelerating its development of a new c-Met-targeted Antibody-Drug Conjugate (ADC). With its newly entered clinical candidate, 'CKD-703,' the company is aiming to secure a best-in-class ADC and is seeking a differentiated strategy in a globally competitive landscape. On August 29, Samsung Medical Center and Aimed Bio co-