- Company
- K-similars won record number of U.S.·Europe nods this year
- by Chon, Seung-Hyun Aug 27, 2024 05:50am
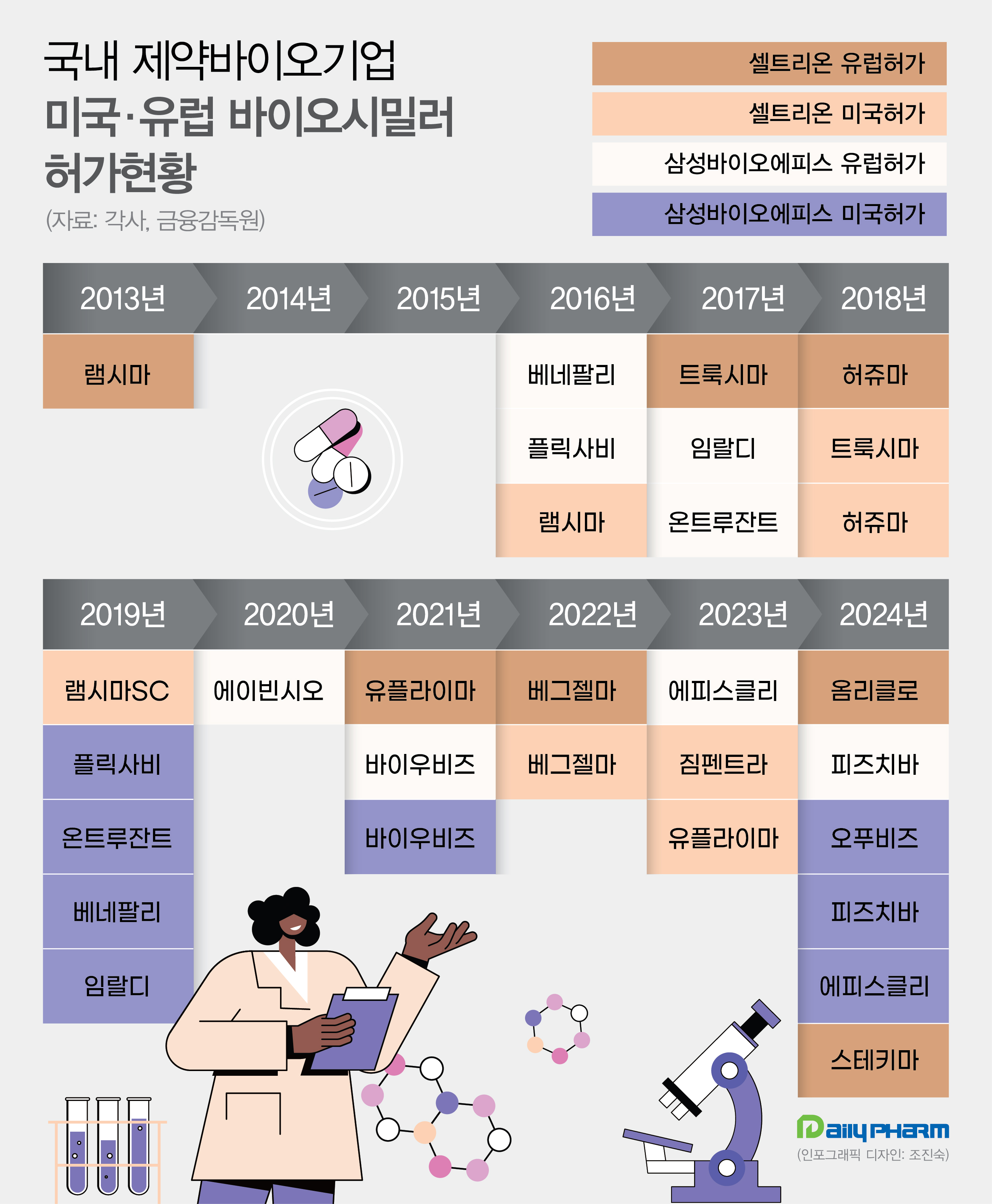
- Korean biopharmaceutical companies have launched the highest number of biosimilars in history this year in the United States and European markets. As Celltrion and Samsung Bioepis won approvals for six biosimilars over eight months in the world's largest market, they topped the previous record of five cases of five years ago. According to sou
- Company
- More urothelial cancer drug options now available in KOR
- by Hwang, Byung-woo Aug 26, 2024 05:46am
- A number of immuno-oncology and antibody-drug conjugate (ADC) drugs are being granted approval as a first-line treatment of urothelial cancer, increasing the treatment options that had previously been dominated by platinum-based chemotherapy. Although they are not yet reimbursed and have yet to become mainstream treatment options, it is expe
- Company
- ‘Lilly will reapply for Retevmo’s reimbursement in Korea’
- by Eo, Yun-Ho Aug 26, 2024 05:46am
- “We will do our best to receive reimbursement for Retevmo in Korea. However, the will of the health authorities is as important” The company has expressed its will to list a RET-targeted therapy option in Korea. The question that remains now is when. Lilly's RET inhibitor Retevmo (selpercatinib) remains non-reimbursed since the compa
- Company
- Colorectal cancer drug Fruzaqla may be introduced in H2
- by Eo, Yun-Ho Aug 26, 2024 05:46am
- A new treatment option is expected to emerge in the field of colorectal cancer later this year. According to industry sources, the final review is underway for the approval of Fruzaqla (fruquintinib), which was designated as a Global Innovative products on Fast Track (GIFT) by the Ministry of Food and Drug Safety in November last year.
- Company
- Expansion of newly launched Kerendia underway in KOR
- by Moon, sung-ho Aug 23, 2024 06:18am
- Since early this year, Kerendia has been covered by reimbursement in clinical practices in South Korea. Kerendia's indication may expand to hypertension as it becomes available for prescriptions at clinical practices. According to the pharmaceutical industry on August 13th, Kerendia (finerenone) is now being used in clinical practices
- Company
- Will the SGLT-2i Jardiance be reimb for CKD in 2H?
- by Eo, Yun-Ho Aug 23, 2024 06:18am
- Attention is focused on whether the SGLT-2 inhibitor Jardiance can settle as a reimbursed prescription option for chronic kidney disease in the second half of the year. According to industry sources, the Health Insurance Review and Assessment Service is currently reviewing the reimbursement expansion of Boehringer Ingelheim and Lilly Kore
- Company
- Yungjin applies for approval of its Ofev generic
- by Hwang, Byung-woo Aug 23, 2024 06:17am
- Yungjin Pharmaceutical announced on the 22nd that it has completed the license application for a generic version of Boehringer Ingelheim's ‘Ofev Soft Cap (Ofev)’ that it had developed by changing the original drug’s soft capsule formulation into a tablet. As a global blockbuster product, Ofev (nintedanib) is widely used in combination
- Company
- Yuhan’s Leclaza enters US 6yrs after signing licensing deal
- by Chon, Seung-Hyun Aug 22, 2024 05:50am
- Yuhan Corp’s new anticancer drug Leclaza has successfully entered the U.S. market. The drug has reached the commercialization stage 6 years after licensing out its technology to Janssen. As a result, the company will receive USD 60 million as a milestone payment with Leclaza’s approval in the U.S. The company has earned nearly KRW 300 billion
- Company
- Bavencio shows clear benefit in urothelial cancer
- by Hwang, Byung-woo Aug 22, 2024 05:50am
- The treatment landscape for metastatic urothelial cell carcinoma has changed after Bavencio (avelumab) was granted reimbursement as a first-line maintenance therapy in Korea. Bavencio addressed the high unmet need that had remained for a maintenance therapy option on site, rapidly changing the prescribing pattern. Merck Biopharma Korea
- Company
- MET-targeting anticancer drug 'Tepmetko' prescribed at Big 5
- by Eo, Yun-Ho Aug 22, 2024 05:50am
- The MET-targeting anticancer drug 'Tepmetko' is now available for prescription at general hospitals. Sources said that Merck Korea's Tepmetko (tepotinib), a treatment for patients with topically advanced or metastatic non-small cell lung cancer (NSCLC) harboring mesenchymal-epithelial transition factor gene exon 14 (METex14) skipping mutat