- Company
- Suhee Shin appointed new General Manager of Amgen Korea
- by Eo, Yun-Ho Jun 19, 2024 05:46am
- Suhee Shin, the former head of Novartis Korea, is coming back to head another Korean subsidiary of a multinational pharmaceutical company after two and a half years. According to industry sources, Director Suhee Shin, who had been leading the Healthcare Innovation Cluster at Roche Korea, has been appointed to take over as the General Mana
- Company
- New multiple sclerosis drug Ocrevus is approved in Korea
- by Son, Hyung-Min Jun 19, 2024 05:46am
- A new drug for multiple sclerosis (MS), an intractable disease characterized by a high relapse rate, has been introduced to the market. Roche's Ocrevus was approved in Korea for the treatment of relapsing-remitting MS as well as primary progressive MS, for which there had been no treatment options available. Medical experts have expressed
- Company
- "Novotech is a global CRO partner for Korean biotech"
- by Lee, Tak-Sun Jun 19, 2024 05:46am
- Novotech is a global full-service clinical Contact Research Organization (CRO) focused on partnering with biotech companies to accelerate the development of innovative and novel therapies at every clinical phase. Recognized for its CRO industry-leading contributions, Novotech received numerous prestigious awards, including the CRO Leadership
- Company
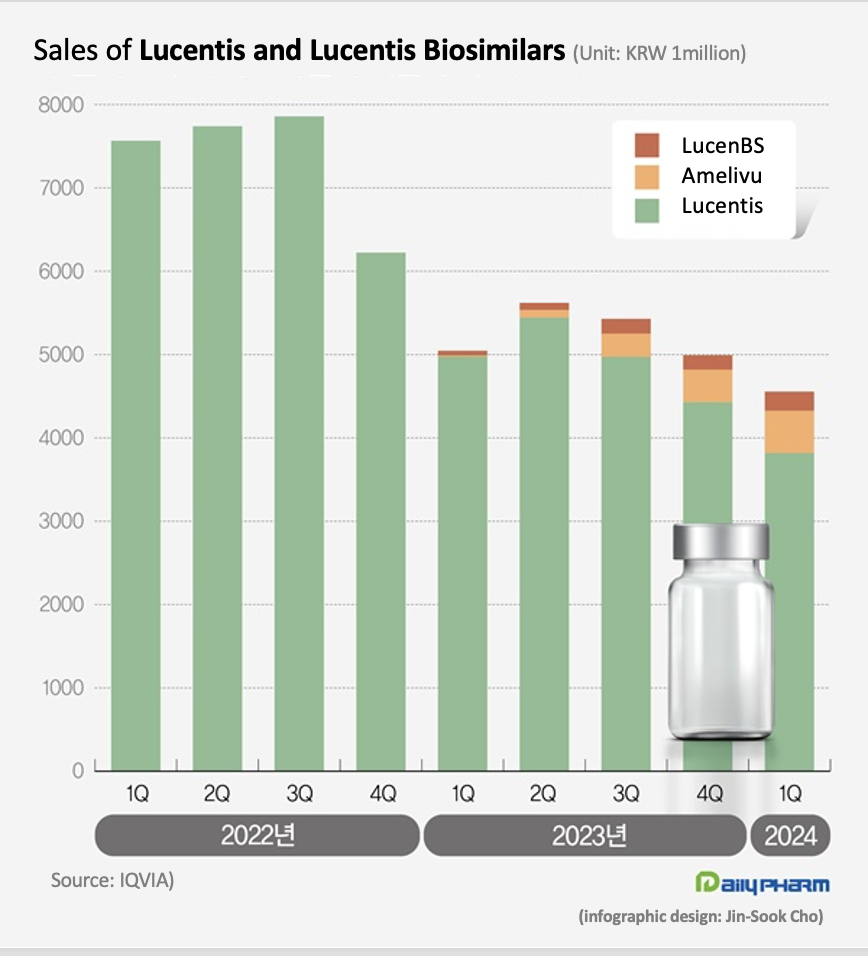
- Lucentis biosimilars occupy 16% of mkt in 1 year
- by Chon, Seung-Hyun Jun 19, 2024 05:46am
- The market size for the eye disease drug Lucentis has shrunk significantly. Last year, its biosimilars entered the market and reduced the drug price of Lucentis, reducing the market size. Although the market share of the two biosimilars remains a mere 16%, the price reduction of Lucentis saved more than KRW 10 billion in annual health insurance
- Company
- Targeted therapies for HER2m NSCLC actively developed
- by Son, Hyung-Min Jun 18, 2024 05:49am
- The domestic and foreign pharmaceutical industry has taken up the challenge of developing a new drug for HER2-positive NSCLC. After Enhertu opened the door by obtaining the first marketing authorization as an anticancer drug targeting the HER2 mutation in Korea, many later entrants such as Yuhan Corp, Boehringer Ingelheim, and Hanmi Pharmaceutic
- Company
- Eisai’s JAK inhibitor, 'Jyseleca,' has landed at hospitals
- by Eo, Yun-Ho Jun 18, 2024 05:48am
- Eisai Korea’s 'Jyseleca,' a JAK inhibitor, is now available for prescription at general hospitals in South Korea. According to industry sources, Jyseleca, which is the fifth JAK inhibitor in South Korea, has passed the drug committee (DC) of Big 5 tertiary general hospitals, including Seoul National University, Seoul Asan Hospital, an
- Company
- Noh will retire from Amgen after 9 years since its inception
- by Eo, Yun-Ho Jun 17, 2024 05:46am
- Noh Sang-kyung (61), general manger of Amgen Korea, who led Amgen’s Korean office since its inception is set to retire from the company. According to industry sources, Noh has recently confirmed his retirement from the company. He has served in this role for nine years since the company’s inception in South Korea in 2015. His succe
- Company
- Elafibranor receives orphan drug designation in Korea
- by Eo, Yun-Ho Jun 17, 2024 05:46am
- The biliary cholangitis drug 'elafibranor' has been designated as an orphan drug in Korea. The Ministry of Food and Drug Safety (MFDS) recently announced the designation through an announcement. Elafibranor, a dual peroxisome-activated peroxisome receptor alpha/delta (PPAR α,δ) agonist that is being developed by Ipsen, received accel
- Company
- Pfizer immediately reapplies to extend reimb for Lorviqua
- by Eo, Yun-Ho Jun 14, 2024 05:47am
- Pfizer Korea has quickly reapplied for reimbursement of Lorviqua, its third-generation ALK anticancer drug whose reimbursement review process recently broke down at the drug pricing negotiation stage. According to Dailypharm’s coverage, Pfizer Korea submitted an application to expand insurance reimbursement for the ALK-positive non-small
- Company
- Industry concern rises over the spread of the medical strike
- by Kim, Jin-Gu Jun 14, 2024 05:46am
- The pharmaceutical industry's situation worsens as the medical community is threatening to take a collective leave of absence due to conflict with the government over increasing medical school admissions. There are voices that say if the leave of absence, which is being made by doctors and some medical professors, spreads throughout the