- Company
- 'Camzyos' anticipated for the DREC review consideration
- by Eo, Yun-Ho May 29, 2024 05:45am
- The industry is awaiting the progress on whether 'Camzyos,' the first novel drug for obstructive hypertrophic cardiomyopathy (oHCM), will receive an insurance reimbursement listing. According to industry sources, BMS Korea’s novel drug Camzyos (mavacamten) has cleared the review by the Economic Evaluation Committee of Health Insurance R
- Company
- Multinational pharmaceutical companies post mixed results
- by Son, Hyung-Min May 28, 2024 01:31pm
- The Korean subsidiaries of multinational pharmaceutical companies posted mixed performances last year. Sales of Pfizer Korea, MSD Korea, and Gilead Sciences Korea, which developed COVID-19 vaccines and treatments, plummeted due to the base effect of the pandemic. On the other hand, GlaxoSmithKline (GSK) and Amgen Korea saw sales increase thanks
- Company
- Handok strengthens global pharma partnerships
- by Kim, Jin-Gu May 28, 2024 05:52am
- Handok is strengthening its partnerships with global pharmaceutical companies. At the end of last year, Handok signed deals with the Dutch company Argenx and Swedish biopharmaceutical company Sobi for three rare disease treatments. This year, the company also launched a combination therapy for high blood pressure co-developed with Sanofi
- Company
- Roche’s Kadcyla sales KRW 70 bil…unrivaled lead in ADC mkt
- by Nho, Byung Chul May 28, 2024 05:52am
- Roche’s Kadcyla continues to remain the unrivaled lead in the domestic ADC (Antibody-Drug Conjugate) drug market, accounting for 60% share of the KRW 120 billion market. Based on drug distribution performance, the domestic ADC drug market was valued at KRW 113.8 billion last year, of which Kadcyla (treatment for HER2-positive metastati
- Company
- ‘Amgen’s success is based on its people and culture’
- by Eo, Yun-Ho May 28, 2024 05:52am
- Amgen is a well-versed player in the industry. Not only is it the leading global biotechnology company, but the company seems to know how to change its form to suit its environment, just like how Amgen Korea adjusts to Korea. Since entering Korea in 2015, Amgen has added 6 of its launched products to the reimbursement list, including its
- Company
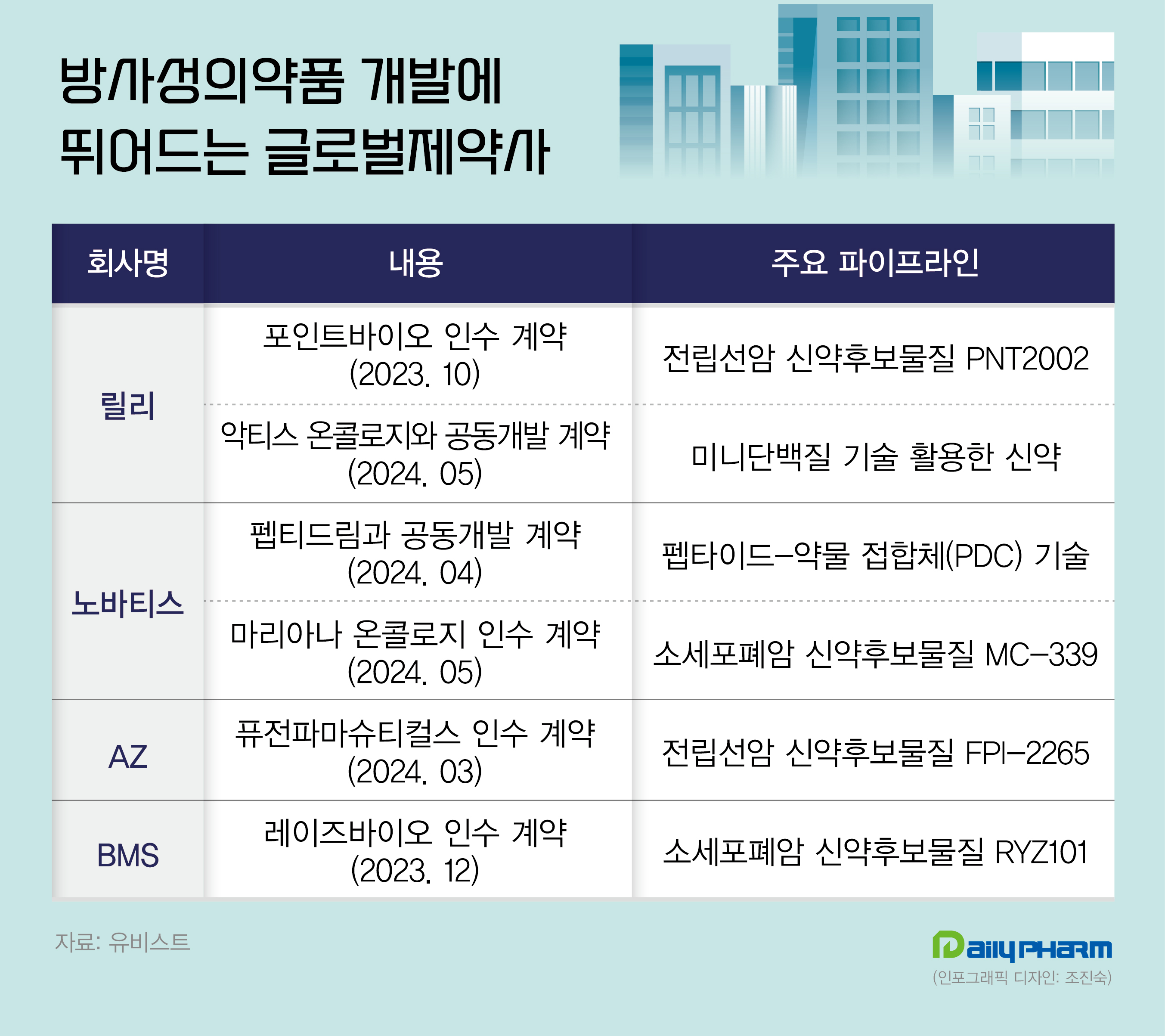
- Global pharmaceutical companies acquire radiopharmaceuticals
- by Son, Hyung-Min May 28, 2024 05:52am
- The high interest in radiopharmaceuticals has led to investments by pharmaceutical companies. Last year and this year, Lily invested KRW 3.5 trillion in a company developing radiopharmaceuticals. Global pharmaceutical companies, including AstraZeneca, Novartis, and BMS, have acquired companies developing radiopharmaceuticals and entered the mark
- Company
- Leclaza demonstrates efficacy as combo therapy in NSCLC
- by Son, Hyung-Min May 27, 2024 05:48am
- Clinical data demonstrating the efficacy of the Leclaza and Rybrevant combination in lung cancer will be presented at the upcoming American Society of Clinical Oncology (ASCO) Annual Meeting. An abstract published for the ASCO 2024 Annual Meeting (ASCO 2024), a five-day conference that is set to start on the 31st, confirmed the additional
- Company
- Bukwang subsidiary’s IPO in Korea will be postponed
- by Kim, Jin-Gu May 24, 2024 05:49am
- Bukwang Pharmaceutical announced that the IPO schedule for its subsidiary Contera Pharma would inevitably need to be postponed. Initially, Contera Pharma planned to gain momentum for its IPO listing in Korea and abroad based on the Phase II results of its new drug candidate for Parkinson's disease, JM-010, but the schedule was postponed a
- Company
- HLB·Jiangsu "FDA, facility issues not related to efficacy"
- by Son, Hyung-Min May 24, 2024 05:48am
- "It appears that there are no issues related to the efficacy of Rivoceranib plus camrelizumab therapy. However, we were pointed out of the manufacturing facility during the monitoring of Jiangsu Hengrui Pharmaceuticals. We do not need an additional clinical trial. By closely cooperating with the U.S. FDA, we will introduce a novel drug to
- Company
- Keytruda posts KRW 100 bil in quarterly sales
- by Chon, Seung-Hyun May 24, 2024 05:48am
- The immuno-oncology drug Keytruda has strengthened its dominance in the domestic drug market, maintaining quarterly sales in the KRW 100 billion range. After being granted reimbursement expansion as a first-line treatment, Keytruda’s sales surged even greater, tripling the sales gap with the runner-up in the market. Also, other new anticancer d