- Company
- Viatris signs exclusive sales and distribution deal for Brid
- by Whang, byung-woo Aug 21, 2025 06:05am
- Viatris Korea announced on the 20th that it has signed an exclusive domestic promotion and distribution agreement for the general anesthesia reversal agent Bridion (Sugammadex) through a strategic partnership with MSD Korea. Under the agreement, Viatris Korea officially took over the domestic promotion and distribution of Bridion as of th
- Company
- Generics challenge the patent of mkt leading 'Rinvoq'
- by Kim, Jin-Gu Aug 21, 2025 06:05am
- The patent challenges by generics targeting AbbVie's Janus kinase (JAK) inhibitor' Rinvoq (upadacitinib)' have begun. The pharmaceutical industry anticipates that patent challenges will further expand as Rinvoq strengthens its monopolistic position in the JAK inhibitor market, which is valued at approximately KRW 62 billion annually.
- Company
- Expanded patent dispute over cancer drug 'Xtandi'
- by Kim, Jin-Gu Aug 20, 2025 06:22am
- The number of companies challenging the patent for Astellas' prostate cancer treatment, 'Xtandi (enzalutamide)', has expanded to six. Attention has been drawn to the fact that major pharmaceutical companies, such as Chong Kun Dang, Hanmi Pharmaceutical, and JW Pharmaceutical, have joined this latest patent challenge. According to the phar
- Company
- Dapa N with Forxiga's indication leads the mkt by surprise
- by Kim, Jin-Gu Aug 20, 2025 06:22am
- Prescription sales of HK inno.N's 'Dapa N,' which received transfer of Forxiga indications, have surged in the SGLT-2 inhibitor diabetes treatment market. It has risen to the top of the dapagliflozin monotherapy market. Analysis suggests that this success is attributed to Dapa N's transition in indications, including chronic heart failu
- Company
- Reimb for 'Padcev' combo as a 1st-line treatment reapplied
- by Whang, byung-woo Aug 19, 2025 06:12am
- Will 'Padcev+Keytruda' combination therapy overcome the reimbursement hurdle in the area of metastatic urothelial cancer, where the first-line treatment option had not been available? In terms of treatment effectiveness, there is no disagreement among experts that, in the long term, it is a first-line standard treatment option. With Astellas
- Company
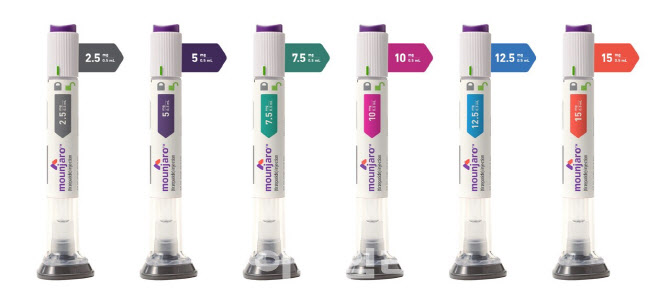
- Will Wegovy patients switch to Mounjaro? Doctors skeptical
- by Moon, sung-ho Aug 19, 2025 06:11am
- With the anticipated launch of Lilly Korea's diabetes and obesity treatment Mounjaro (tirzepatide) in mid-August, its full-scale competition with Novo Nordisk's Wegovy (semaglutide) is expected to unfold. Amid the imminent competition, the issue of whether to switch between the two drugs has emerged as a key point of contention in clinical se
- Company
- PARP inhibitor 'Lynparza' reattempts prostate cancer reimb
- by Eo, Yun-Ho Aug 18, 2025 06:03am
- Attention is focused on whether there will be progress in the expanded reimbursement of the PARP inhibitor 'Lynparza' for prostate cancer. According to industry sources, AstraZeneca Korea's PARP (Poly ADP-ribose polymerase) inhibitor Lynparza (olaparib) is expected to be considered for the Health Insurance Review & Assessment Service (HIRA
- Company
- Four CAR-T therapies to compete for 'Kymriah's mkt position'
- by Whang, byung-woo Aug 18, 2025 06:02am
- With the third new CAR-T therapy, 'Yescarta,' obtaining marketing authorization in Korea, market competition for treating relapsed or refractory Diffuse Large B-cell Lymphoma (DLBCL) is anticipated. With Kymriah (tisagenlecleucel) from Novartis Korea being the only option currently reimbursed, the impact of Yescarta, which has shown prominen
- Company
- New Praluent dosage form available at general hospitals
- by Eo, Yun-Ho Aug 18, 2025 06:02am
- A new dosage form of the PCSK9 inhibitor ‘Praluent’ may be prescribed at general hospitals in Korea. According to industry sources, a pen formulation of Sanofi-Aventis Korea's primary hypercholesterolemia and mixed dyslipidaemia treatment Praluent (alirocumab), Praluent Pen 300mg inj, has been approved by the Drug Committees (DCs) of te
- Company
- Will Korean companies join in on the sales of obesity drugs?
- by Kim, Jin-Gu Aug 18, 2025 06:02am
- With the domestic launch of the GLP-1 class obesity treatment Mounjaro (tirzepatide) near, its sales and marketing competition with Wegovy (semaglutide) is already heating up. Lilly has completed preparations for the launch by hiring over 30 additional medical and marketing representatives to handle sales of Mounjaro. Additionally, the po