- Company
- Only 1 of 10 CEOs ‘positive’ about drug regulations in KOR
- by Chon, Seung-Hyun Jan 3, 2024 07:28pm
- A survey showed only 1 in 10 CEOs of pharmaceutical companies were found to be satisfied with the Korean health authorities' regulatory policies for the pharmaceutical industry. In all major regulatory areas such as approvals, drug pricing, and sales, the satisfaction rate of CEOs was more negative than positive towards the set regulations.
- Company
- New antibiotic to be listed for reimb, following Zerbaxa
- by Eo, Yun-Ho Jan 3, 2024 05:40am
- Zavicefta, the next-generation antibiotic, is progressing towards reimbursement listing. According to the industry, Pfizer Pharmaceutical Korea is currently in the final round of discussions with the National Health Insurance Service (NHIS) to settle the reimbursement pricing negotiations for its Zavicefta Inj (ceftazidime·avibactam).
- Company
- ‘Drug pricing and reimb' needs most regulatory improvement
- by Chon, Seung-Hyun Jan 3, 2024 05:40am
- CEOs of both domestic and multinational pharmaceutical companies alike identified 'drug prices and reimbursement as in need of most regulatory improvement. CEOs of multinational pharmaceutical companies only gave a 2-point range satisfaction score in terms of satisfaction with Korea’s drug pricing and reimbursement regulations. This indica
- Company
- SK Chemicals ends copromotion agreement with Eli Lilly
- by Lee, Tak-Sun Jan 2, 2024 05:45am
- SK Chemicals announced that its copromotion agreement with Lilly Korea for the antidepressant Cymbalta Cap (duloxetine hydrochloride) and the migraine treatment Emgality (galcanezumab-gnlm) has been brought to an end. Cymbalta is the number one selling antidepressant on the market. Sales of Emgality have also been rising since last year'
- Company
- CMV treatment Livtencity lands in Big 5 hospitals in KOR
- by Eo, Yun-Ho Jan 2, 2024 05:45am
- Livtencity, a cytomegalovirus (CMV) treatment, is nearing prescription at tertiary general hospitals. According to industry sources, Takeda Pharmaceuticals Korea’s Livtencity (maribavir) has recently passed the Drug Committee (DC) of the “Big 5” hospitals, including Seoul National University Hospital, Samsung Seoul Hospital, Seoul St
- Company
- Celltrion merges and launches with founder’s son as leader
- by Kim, Jin-Gu Dec 29, 2023 05:40am
- Celltrion’s Chair of the Board, Jin-Seok Seo, the eldest son of Celltrion’s Chairman Emeritus Jung-jin Seo, will helm the newly merged Celltrion. The new CEO Seo will lead the company in a 3-person representative system with Hyung-Ki Kim, current CEO of Celltrion, and Hyung-Ki Kim, former CEO of Celltrion Healthcare. Celltrion annou
- Company
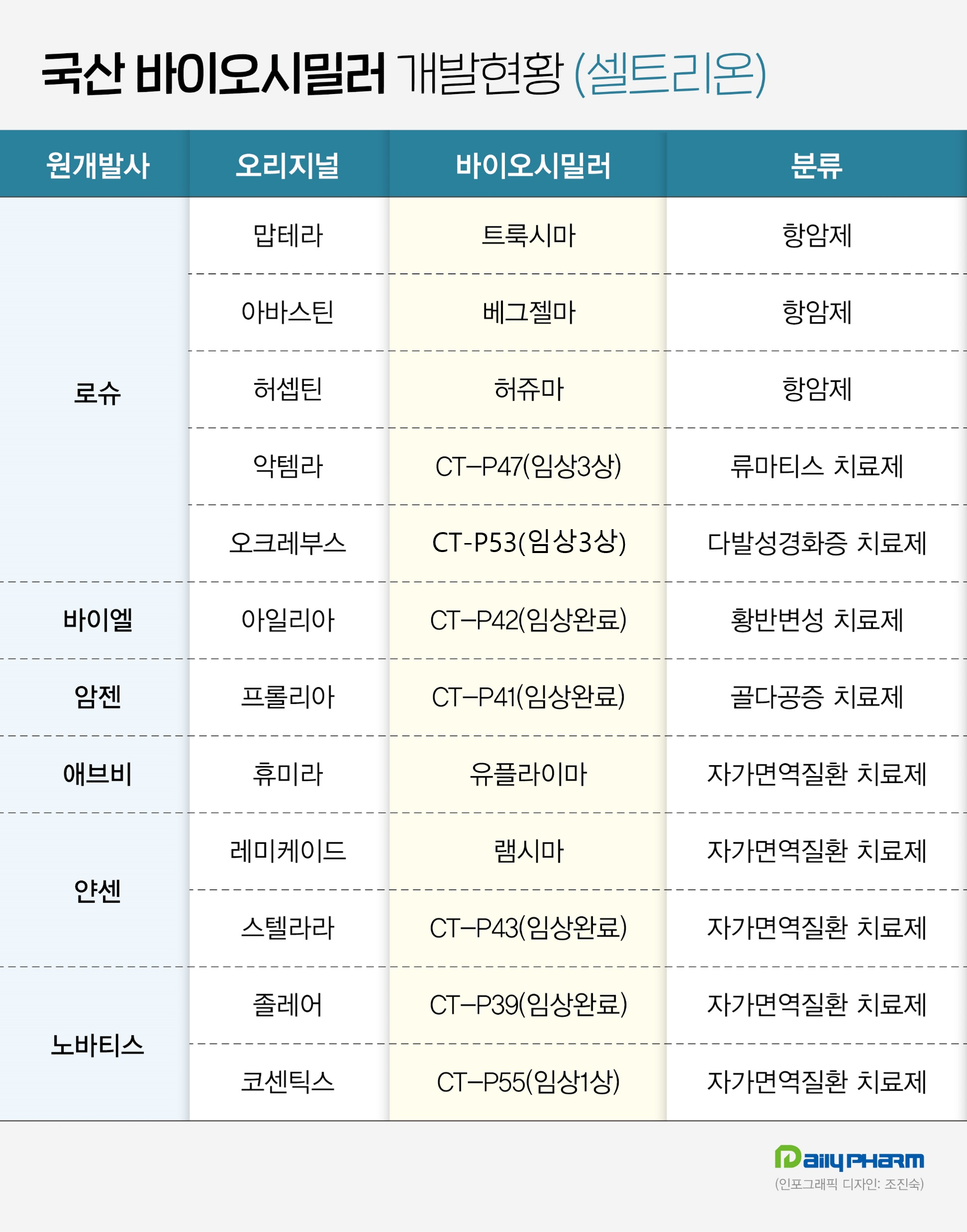
- K-similars set out to enter the global market
- by Son, Hyung-Min Dec 29, 2023 05:40am
- Domestic companies are now ready to launch homegrown biosimilars into the global market next year. According to industry sources on the 28th, Celltrion, Samsung Bioepis, and Dong-A ST have completed Phase III clinical trials of its biosimilars and applied for approval from overseas regulators. The companies have successfully developed biosimi
- Company
- Integrated Celltrion launches with goal of 40T market cap
- by Kim, Jin-Gu Dec 29, 2023 05:39am
- Celltrion and Celltrion Healthcare are set to launch an integrated corporation. The market capitalization of the integrated Celltrion is expected to reach around 40 trillion won. The launch of the integrated Celltrion is anticipated to unify the previously dispersed biosimilar production and distribution processes. This merger may addre
- Company
- Medytox submits BLA to FDA for its next-gen botulinum
- by Chon, Seung-Hyun Dec 29, 2023 05:39am
- Medytox is working to enter the U.S. market with its next-generation botulinum toxin product, which technology has been reverted to the company after being transferred to a foreign company. On the 27th, Medytox announced that it has filed a biological license application (BLA) to the U.S. Food and Drug Administration (FDA) for its next-gen
- Company
- Hugel Resolves to Retire 371,563 shares of its own stock
- by Nho, Byung Chul Dec 28, 2023 01:14pm
- Hugel, a global total medical aesthetics company, announced that it has held a board of directors meeting on the 22nd and passed a resolution to retire 371,563 shares of its own stock to enhance the shareholder’ value. This is equivalent to around 3% of the total outstanding shares (12,385,455 shares), and the expected value of the retired s