- Company
- "Winrevair, gamechanger for pulmonary arterial hypertension"
- by Whang, byung-woo Aug 18, 2025 06:01am
- Winrevair (sotatercept), a new mechanism treatment for pulmonary arterial hypertension that has emerged after over 20 years, has been approved in Korea. Consequently, competitive market is expected. Although reimbursement remains a challenge, it is expected to change the treatment paradigm for pulmonary hypertension, a disease with significan
- Company
- Imfinzi denied reimb for bile duct cancer for the 3rd year
- by Eo, Yun-Ho Aug 14, 2025 06:13am
- Attention is focused on whether there will be progress in the reimbursement process for Imfinzi for bile duct cancer, which has remained non-reimbursed for three years now. According to industry sources, the Health Insurance Review and Assessement Service's Drug Reimbursement Evaluation Committee may likely review the agenda of expanding
- Company
- "A paradigm shift in atopic dermatitis treatment"… Adtralza
- by Whang, byung-woo Aug 14, 2025 06:13am
- The treatment landscape is undergoing significant changes as new drugs for atopic dermatitis, including biologics and JAK inhibitors, have emerged over the past few years. Experts assess that patients who gave up on receiving treatments due to recurrence and side effects are revisiting hospitals with the introduction of new drugs. As treat
- Company
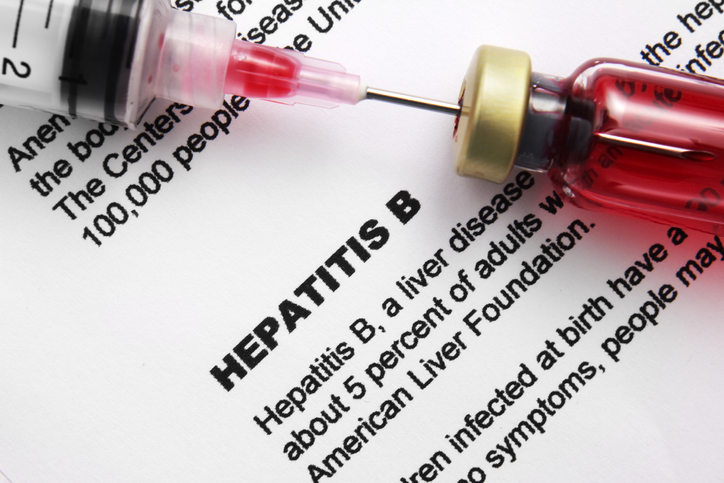
- Only Vemlidy’s sales grew in the ₩300B HBV drug mkt
- by Kim, Jin-Gu Aug 14, 2025 06:13am
- Korea's hepatitis B treatment market, which is valued at KRW 300 billion annually, is rapidly restructuring around Vemlidy (tenofovir alafenamide). Among original products, only Vemlidy is maintaining its growth, and in the generic market, only the Vemlidy series has shown a significant increase in sales. HBV treatment market reaches KRW
- Company
- 'Tevimbra' applies for reimbursement for five indications
- by Eo, Yun-Ho Aug 13, 2025 06:07am
- 'Tevimbra' is quickly expanding following its successful inclusion in the insurance reimbursement for esophageal cancer. BeOneMedicines Korea has been confirmed to have applied for reimbursement on the day of approval for five new indications for its PD-1 inhibitor immunotherapy Tevimbra (tislelizumab), which received additional approval
- Company
- Adult vaccination in a super-aged society highlighted
- by Whang, byung-woo Aug 13, 2025 06:07am
- South Korea has entered a super-aged society, with its population aged 65 and over exceeding 20%. The need for a response through adult vaccination is being emphasized. With the number of National Immunization Program (NIP) vaccines for adults limited to just influenza and pneumococcal vaccines, there is a growing opinion that the program nee
- Company
- Tariff impact inevitable for biodrugs’ export to the US
- by Cha, Jihyun Aug 12, 2025 06:16am
- Exports of domestically produced biopharmaceuticals to the US more than doubled last year compared to the previous year. If the Trump administration's tariffs on pharmaceuticals are implemented, the domestic biopharmaceutical export industry is expected to suffer significant damage. According to the “Key Data on the Domestic Biopharmaceutica
- Company
- 'Vanrafia' for rare kidney disease receives ODD in Korea
- by Eo, Yun-Ho Aug 12, 2025 06:16am
- A new drug for rare kidney disease, 'Vanrafia,' has been granted orphan drug designation in Korea. The Ministry of Food and Drug Safety (MFDS) recently announced this through notificiation board. The indication for the designation is to 'treat adult patients with primary immunoglobulin A nephropathy (IgAN) with a urine protein-to-creatin
- Company
- US Biosecurity Act passing is ramping up
- by Kim, Jin-Gu Aug 12, 2025 06:14am
- The U.S. Congress is re-pursuing the Biosecurity Act, which failed to pass last year. The bill explains a comprehensive set of sanctions against companies designated as 'biotechnology companies of concern.' Analysis suggests that the bill's chances of passing have increased, as it addresses the procedural issues that were a stumbling bl
- Company
- Gilead’s Livdelzi receives orphan drug designation in KOR
- by Eo, Yun-Ho Aug 11, 2025 06:05am
- The new primary biliary cholangitis drug ‘Livdelzi’ has received the orphan drug designation in Korea. The Ministry of Food and Drug Safety announced the news in a public notice on the 4th. Specifically, the designated indication is “the treatment of primary biliary cholangitis (PBC) in adults who have an inadequate response or intoler