- Company
- Samsung Biologics signs 2 CMO contracts with Pfizer
- by Chon, Seung-Hyun Jul 5, 2023 05:44am
- Samsung Biologics signed two contract manufacturing organization agreements with Pfizer. The contracts, including the largest single contract ever made that is valued at KRW 922.7 billion (approximately USD 818 million), have been successfully concluded, totaling the amount to KRW 1.2 trillion (approximately USD 1.06 billion). On the 4th
- Company
- Samsung Bioepis launches Humira biosimilar in the US
- by Lee, Seok-Jun Jul 4, 2023 05:37am
- On July 3rd, Samsung Bioepis announced that it had released its Humira biosimilar ‘Hadlima (project name: SB5, ingredient: adalimumab) in the US through its partner Organon. Hadlima is a treatment for autoimmune diseases including rheumato
- Company
- Celltrion seeks approval for Stelara biosimilar in the US
- by Kim, Jin-Gu Jul 4, 2023 05:37am
- Celltrion announced on the 3rd that it has submitted an application for the approval of its Stelara (ustekinumab) biosimilar to the U.S. Food and Drug Administration (FDA). Its indications are the same as its original - plaque psoriasis, pediatric plaque psoriasis, psoriatic arthritis, pediatric psoriatic arthritis, Crohn's disease, and u
- Company
- Discussion on whether Vabysmo benefit is possible
- by Eo, Yun-Ho Jul 4, 2023 05:37am
- Attention is focusing on whether Vabysmo, a macular degeneration treatment, will be able to be listed on the insurance benefit list. According to related industries, it is expected that Vabysmo, a Bispecific antibody treatment in Roche Korea, will be presented to the Drug Benefit Evaluation Committee this month (July). This drug passed the Dr
- Company
- Forxiga expands its benefit through heart failure
- by Jung, Sae-Im Jul 4, 2023 05:36am
- Domestic and foreign cardiac societies recommend SGLT-2 for preserving exudation rates Forxiga, a blockbuster treatment with an annual prescription of 90 billion won, has expanded its scope to the entire heart failure. AstraZeneca Korea held a press conference at The Plaza Hotel in Jung-gu, Seoul on the 3rd to commemorate the expansion of chron
- Company
- Lagevrio was voluntarily withdrawn from Europe
- by Kim, Jin-Gu Jul 3, 2023 05:47am
- Merck has voluntarily withdrawn its European approval application for Lagevrio, an oral COVID-19 treatment. The pharmaceutical industry is paying close attention to whether the global supply of this drug will continue. Celltrion and Hanmi Pharmaceutical, which had already decided to produce and supply Lagevrio generics to developing countrie
- Company
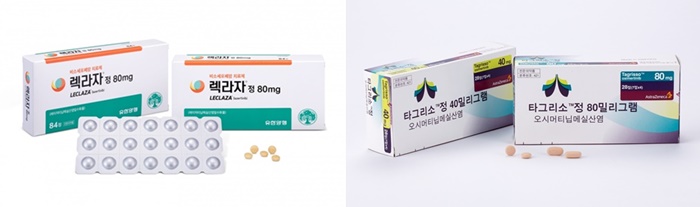
- Leclaza and Tagrisso compete for reimb in the first line
- by Jung, Sae-Im Jul 3, 2023 05:47am
- Yuhan Corp’s EGFR-targeted anticancer drug ‘Leclaza (lasertinib)’ has been approved as a first-line treatment in Korea and is competing for reimbursement with its competitor ‘Tagrisso (osimertinib).’ As reimbursement review for Tagrisso’s first-line indication is already in progress, Yuhan is also expected to hasten its steps to re
- Company
- Pfizer, Lilly, AbbVie engage in JAKi battle in KOR
- by Nho, Byung Chul Jul 3, 2023 05:47am
- Pfizer's Xeljanz and Lilly's Olumiant are engaged in an intense competition for sales in the JAK inhibitor market. According to the distribution data of pharmaceuticals, Xeljanz (including Xeljanz XR) recorded sales of KRW 15.5 billion won last year, while Olumiant recorded KRW 15.4 billion. Although Xeljanz maintained a slight lead,
- Company
- Illaris for 13 pts, re-challenge to enter insurance benefit
- by Eo, Yun-Ho Jul 3, 2023 05:45am
- Illaris, a treatment for vulva diseases with only 13 patients in Korea, will try again to enter the insurance benefit zone. According to related industries, Novartis Korea submitted an application for Ilaris' benefit in April and is under discussion. However, the registration process is still expected to be difficult. The drug has already und
- Company
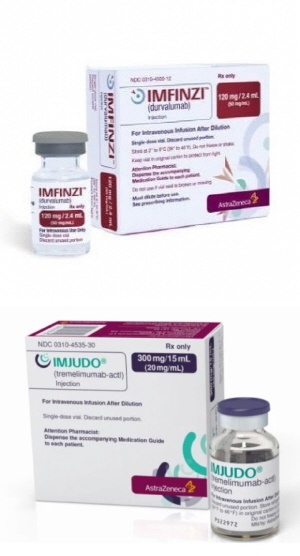
- Imfinzi+Imjudo combo approved for liver cancer in KOR
- by Eo, Yun-Ho Jul 3, 2023 05:45am
- The immuno-oncology drug ‘Imfinzi’ added an indication in Korea and can be used in combination with ‘Imjudo’ in Korea. According to industry sources, AstraZeneca received approval for the combined use of Imfinzi+Imjudo from the Ministry of Food and Drug Safety on June 30th, immediately after receiving approval for its CTLA-4 inhibitin