- Company
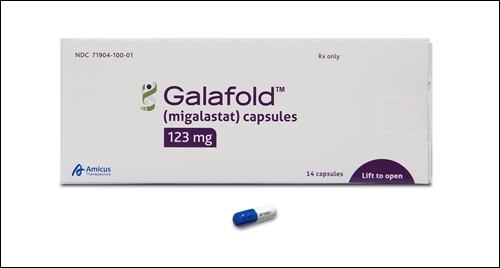
- Galafold reimbursed as first-line drug for Fabry disease
- by Nho, Byung Chul Aug 4, 2025 05:54am
- Reimbursement for Handok's Fabry disease treatment, Galafold (migalastat), will be expanded to cover its use as a first-line treatment starting on the first of this month. Previously, Galafold was covered only for patients aged 16 and older who had been administered enzyme replacement therapy (ERT) intravenously for at least 12 months.
- Company
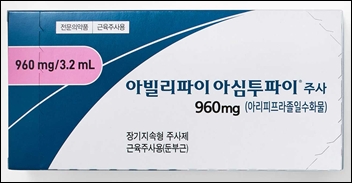
- Otsuka launches once-every-2-months inj 'Abilify Asimtufii'
- by Whang, byung-woo Aug 4, 2025 05:54am
- Otsuka Pharmaceutical Korea announced on August 1 that it will launch 'Abilify Asimtufii Inj (aripiprazole monohydrate),' starting in August, as the reimbursement coverage by the National Health Insurance has been applied. 'Abilify Asimtufii Inj' is an extended-release formulation administered once every two months. It was approved on Feb
- Company
- Integrated CKM approach, the new paradigm for CKD treatment
- by Whang, byung-woo Aug 4, 2025 05:53am
- “In the past, we relied solely on single agents such as ACE inhibitors or ARBs to treat chronic kidney disease, but now an integrated approach that simultaneously manages cardiovascular, renal, and metabolic conditions is rising as the option. In this context, the results of the CONFIDENCE study, a recent study on the early combined use of SGLT
- Company
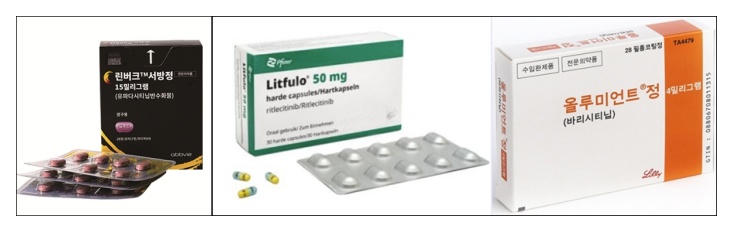
- Rinvoq joins the competition in the alopecia areata trt. mkt
- by Whang, byung-woo Aug 4, 2025 05:53am
- AbbVie's JAK inhibitor Rinvoq (upadacitinib) is expected to enter the market competition following successful clinical trials for alopecia areata. AbbVie recently announced positive topline results from the No.2 study of the Phase 3 clinical program (UP-AA program) for Rinvoq in adult and adolescent patients with severe alopecia areata.
- Company
- Janssen, Pharma distribution industry agree on margin adj
- by Son, Hyung Min Aug 1, 2025 06:18am
- The conflict between Janssen Korea and the pharmaceutical distribution industry over distribution margin reductions has been resolved with a negotiated settlement. The distribution industry achieved an outcome that maintains existing trade relationships, including with small and medium-sized distributors, while also improving the proposed mar
- Company
- NMOSD drug Enspryng’s reimbursement expanded in KOR
- by Whang, byung-woo Aug 1, 2025 06:16am
- Roche Korea announced on the 31st that the reimbursement criteria for Enspryng (satralizumab), a treatment for neuromyelitis optica spectrum disorder (NMOSD), will be expanded from August 1st per a notification from the Ministry of Health and Welfare. Enspryng is indicated for the treatment of adult patients with anti-aquaporin(AQP4) anti
- Company
- Roche's HR+ breast cancer drug 'Itovebi' wins nod in Korea
- by Whang, byung-woo Aug 1, 2025 06:15am
- Roche Korea announced on July 30 that it has received approval of the breast cancer treatment Itovebi (inavolisib) from the Ministry of Food and Drug Safety. Itovebi, recently approved, can be used for the treatment of PIK3CA mutation-positive, hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2-) breas
- Company
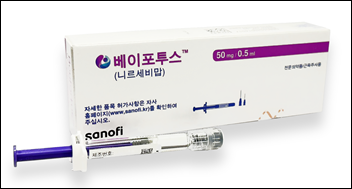
- Beyfortus likely to face restrictions on public advertising
- by Whang, byung-woo Jul 31, 2025 06:15am
- Sanofi is now compelled to revise its strategy as public advertising of Beyfortus (nirsevimab), an RSV preventive antibody injection, faces regulatory obstacles in Korea. According to Dailypharm’s coverage, the&160;Korea Pharmaceutical and Bio-Pharma Manufacturers Association (KPBMA) Drug Advertising Review Committee ruled that Beyfortu
- Company
- MSD Korea begins Januvia drug price difference compensation
- by Son, Hyung Min Jul 31, 2025 06:14am
- There are changes regarding the Januvia drug price difference compensation issue, which had been stalled for nearly two years. MSD Korea has officially informed the distribution industry that it will begin accepting applications for Januvia compensation starting next month, August 1. However, this compensation is limited to the quantity sold
- Company
- GSK Korea appoints Gunnar Riediger as new General Manager
- by Whang, byung-woo Jul 31, 2025 06:14am
- GSK Korea announced on the 30th that it has appointed Gunnar Riediger as its new General Manager, effective August 1. The new GM joined GSK in 2004 through the company's global talent development program, the “Future Leaders Program,” and has led healthcare operations across Latin America for over 20 years. He has held key roles incl