- Company
- Neurophet partners with Roche for AI tech verification
- by Whang, byung-woo Jul 30, 2025 06:10am
- Neurophet (Co-CEO Jun-gil Bin, Dong-hyun Kim), a specialized AI company in brain disease diagnosis and treatment, announced on the 29th that it has officially signed a joint research agreement with Roche and has begun full-scale collaboration Neurophet has already been sharing data with Roche, and with the official announcement of the col
- Company
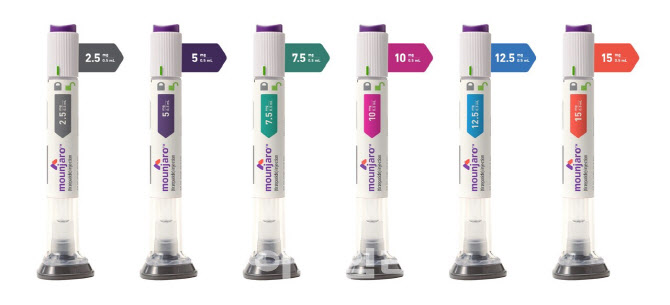
- Attention drawn to reimb status of 'Mounjaro' for diabetes
- by Moon, sung-ho Jul 30, 2025 06:09am
- As the official launch of Mounjaro (tirzepatide, Eli Lilly Korea), approved in Korea as an adjunct therapy for chronic weight management following its indication for adult type 2 diabetes, is imminent, the reimbursement status of the drug is garnering attention. The focus is on whether it will be recognized and reimbursed as the first 'innova
- Company
- "A paradigm shift in ulcerative colitis treatment"
- by Whang, byung-woo Jul 29, 2025 06:04am
- The treatment strategy for ulcerative colitis is rapidly evolving with the emergence of new global drugs. Global guidelines recommend a rapid transition to advanced therapies (ATs) early on if 5-ASA treatment fails. In line with these recommendations, Korea also needs a shift in its treatment paradigm. However, the early utilization of hig
- Company
- Fewer drugs reimbursed 5 years into pricing reform
- by Chon, Seung-Hyun Jul 29, 2025 06:04am
- Over the past five years, 4,500 drugs have been removed from the reimbursement list in Korea. The number of new drugs entering the market has decreased significantly with the introduction of generic drug price reforms and joint development regulations. The number of prescription drug approvals has decreased by more than 80% compared to 5 years a
- Company
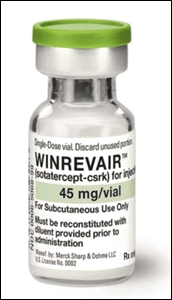
- 1st drug to win 2nd concurrent approval·pricing program
- by Eo, Yun-Ho Jul 28, 2025 06:08am
- As the first drug has been approved under the 2nd concurrent approval·drug pricing program, the process for insurance reimbursement is garnering attention. MSD Korea's 'Winrevair (sotatercept),' a new drug for pulmonary arterial hypertension (PAH), recently obtained final approval from the Ministry of Food and Drug Safety. As it has be
- Company
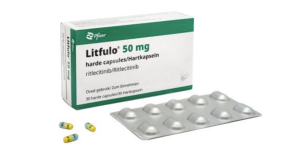
- Alopecia areata drug Litfulo lands in the Big 5 hospitals
- by Eo, Yun-Ho Jul 28, 2025 06:07am
- Litfulo, a new drug for alopecia areata, may now be prescribed at tertiary hospitals in Korea.. According to industry sources, Pfizer Korea's new Janus kinase (JAK) inhibitor Litfulo (ritlecitinib) has passed review of Drug Committees (DCs) at major medical institutions, including Samsung Medical Center, Seoul National University Hospital
- Company
- Mounjaro launch set for August in Korea
- by Whang, byung-woo Jul 25, 2025 06:11am
- The launch date for Mounjaro (tirzepatide), a dual GIP/GLP-1 receptor agonist, has been set, signaling the start of full-fledged competition in the obesity treatment market in Korea. Lilly Korea announced on the 23rd that it will launch Mounjaro 2.5mg and 5mg/0.5mL in mid-August for patients with type 2 diabetes and obesity in Korea. Mo
- Company
- Verzenio fails to pass third CDDC review for reimb in KOR
- by Eo, Yun-Ho Jul 25, 2025 06:10am
- Unfortunately, the third time was not the charm. The breast cancer treatment Verzenio is facing difficulties in expanding insurance reimbursement for early breast cancer in Korea. On the 23rd, Lilly Korea’s CDK4/6 inhibitor Verzenio (abemaciclib), which sought to secure reimbursement for its early breast cancer indications, failed to pas
- Company
- Expanded reimbursement criteria for 'Enspryng'
- by Eo, Yun-Ho Jul 24, 2025 06:07am
- As the insurance reimbursement criteria for 'Enspryng' have been eased, the drug is expected to be more utilized. The Ministry of Health and Welfare (MOHW) recently announced an administrative announcement regarding the partial revision to the "Detailed Standards and Methods of Application for Health Insurance Benefits (Pharmaceuticals),"
- Company
- Wegovy legend Novo Nordisk pursues public good and profit
- by Cha, Jihyun Jul 24, 2025 06:06am
- KRW 318 trillion. This is the market capitalization of Novo Nordisk as of the closing price on the 16th. The market capitalization of this single Danish pharmaceutical company is more than 15 times larger than the combined market capitalization of the top 10 pharmaceutical companies in South Korea (JW Pharmaceutical, Kwangdong Pharmaceutic