- Company
- Merz’s OTC drug Pantogar released as health functional food
- by Nho, Byung Chul Jun 9, 2021 06:08am
- The hair loss OTC drug Pantoga received attention after its unexpected release as a health functional food recently, after removing some of its ingredients. Pantogar was approved by the Korean Ministry of Food and Drug Safety in 2002, and the original developer, Merz in Germany, had globally launched Pantogar in 1976. The newly launche
- Company
- MNCs bid for ‘themed’ victory with innovation and legacy
- by Eo, Yun-Ho Jun 9, 2021 06:07am
- Whichever the cause, the decision of the Big Pharmas to ‘focus on their strengths’ is more than just a pretext for reorganizing their businesses. Setting a clear ‘theme’ can have a positive effect on the image of a company. Also, dividing its business does not mean a loss of drive. However, such divisions will bring one company to los
- Company
- Patent dispute over Pelubi SR is not over
- by Kim, Jin-Gu Jun 9, 2021 06:07am
- The patent dispute of Daewon's anti-inflammatory drug Pelubi SR continues. Youngjin, Huons, and Chong Kun Dang won patent dispute of Pelubi. Among them, Youngjin has received generic for exclusivity alone. According to the pharmaceutical industry on the 7th, at least three companies are considering patent challenges for Pelubi SR: Mothers
- Company
- Hemlibra reimbursement disapproval raises anxiety
- by Nho, Byung Chul Jun 8, 2021 06:01am
- The head-on clash between the ‘standard to first consider immune tolerance induction (ITI·antibody removal) therapies’ and the ‘due prescription rights of doctors·convenience in administration·saving NHI finances’ have received industry attention. On the 3rd, the Health Insurance Review and Assessment Service (HIRA) held a Pediatri
- Company
- Pros and cons of the spin-offs and sales of Big Pharmas
- by Eo, Yun-Ho Jun 8, 2021 06:01am
- Mergers, spin-offs, buying, selling... news shows that global Big Pharmas have been busy constantly changing their shape. In particular, the issue that gained the most attention for the past few years was the companies' spin-offs and sales. Although the companies' made the decision under the premise of ‘focusing on one’s strengths,’ s
- Company
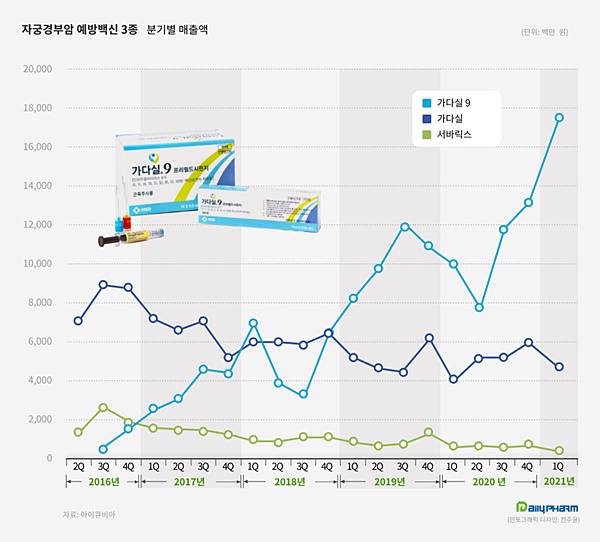
- HPV vaccine market expands 55% midst COVID-19
- by An, Kyung-Jin Jun 7, 2021 06:06am
- The market for the vaccine to prevent cervical cancer has expanded by the greatest amount ever. The increased inoculation rate of the high-priced 'Gardasil 9' has led the total market growth. MSD, which owns two HPV vaccines - 'Gardasil' and 'Gardasil 9' - accounted for 97% of the total market, and boasted its overwhelming influence in t
- Company
- Sales in the herpes zoster market have halved in 2 years
- by An, Kyung-Jin Jun 4, 2021 06:06am
- Sales in the domestic shingles prevention vaccine market, which had been on the mend, fell again. In December last year, sales of two vaccines to prevent shingles fell as the vaccination rate fell in the wake of the third pandemic of COVID-19. According to IQVIA, a pharmaceutical research institute, the size of the vaccine market for shingles
- Company
- Roche Korea conducts the voluntary retirement program
- by Jun 3, 2021 05:56pm
- Roche Korea conducts the Early Retirement Program (ERP). In the first half of this year alone, many multinational pharmaceutical companies, including Viatris, Astellas, and GSK, started reducing the number of people. According to pharmaceutical industry on the 3rd, Roche Korea is currently conducting ERP for reorganization. The first targ
- Company
- Amgen speeds up commercialization of its KRAS drug
- by Eo, Yun-Ho Jun 3, 2021 06:12am
- Amgen is rapidly working to commercialize its KRAS targeted anticancer therapy in Korea. Industry sources have said that Amgen Korea has submitted an application for the marketing authorization of the first-ever KRAS-targeted anticancer therapy, ‘Lumakras (sotorasib),' on the 28th to the Korean Ministry of Food and Drug Safety (MFDS) imm
- Company
- Awareness of multiple sclerosis should increase
- by Jun 3, 2021 06:12am
- Today (26th) is World MS Day. Although the treatment environment has improved significantly with the release of more new drugs for multiple sclerosis compared to the past, early diagnosis is not easy due to the low awareness of the disease. The same is true of overseas situations. In response, the International Association for Multiple Sclero