- Company
- Trodelvy may be the 1st drug reimbursed with ICER benefits
- by Eo, Yun-Ho May 21, 2025 06:36am
- ADC breast cancer drug Trodelvy may soon be listed for insurance reimbursement in Korea. Gilead Sciences recently finalized price negotiations with the National Health Insurance Service for its triple-negative breast cancer (TNBC) treatment Trodelvy (sacituzumab govitecan). As a result, Trodelvy is scheduled to be presented at the Heal
- Company
- MSD expands domestic clinical trial cooperation
- by Whang, byung-woo May 20, 2025 06:00am
- MSD Korea has broken its record for the most clinical trial approvals in Korea and is now in full swing, developing innovative new drugs for Koreans. With open innovation playing an increasingly important role in new drug research and development (R&D), MSD is expanding its ties with Korea, which plays a pivotal role in its global clinical tr
- Company
- Hanmi-MSD collaborate for R&D
- by Cha, Jihyun May 20, 2025 05:59am
- Hanmi Pharmaceutical entered into a clinical trial collaboration agreement with the U.S.-based Merck (MSD) for developing an immune anticancer drug candidate. The clinical collaboration between Hanmi Pharmaceutical (hereafter, Hanmi) and Merck has expanded to three cases. In addition to clinical trial collaboration, Hanmi continues to collaborat
- Company
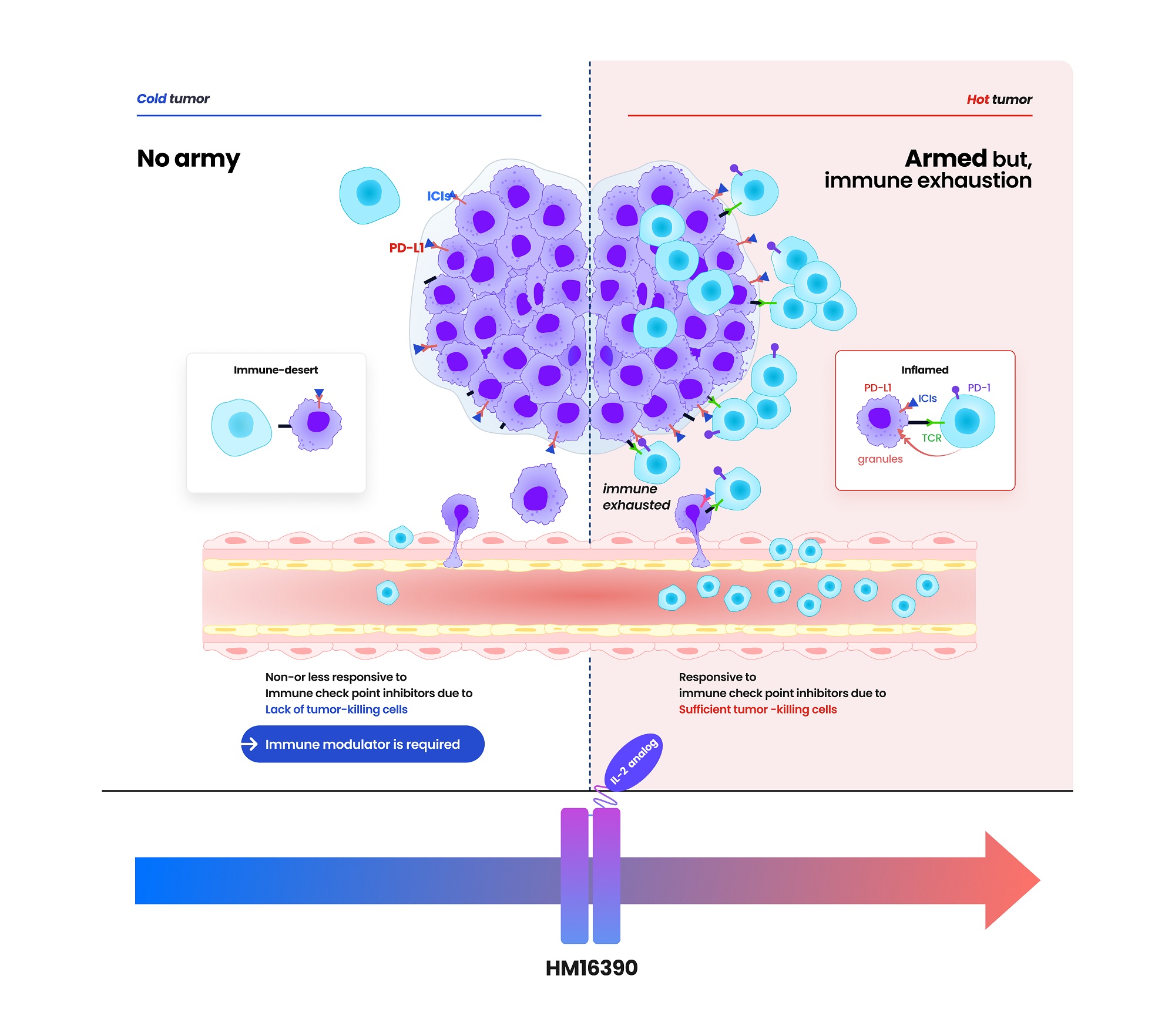
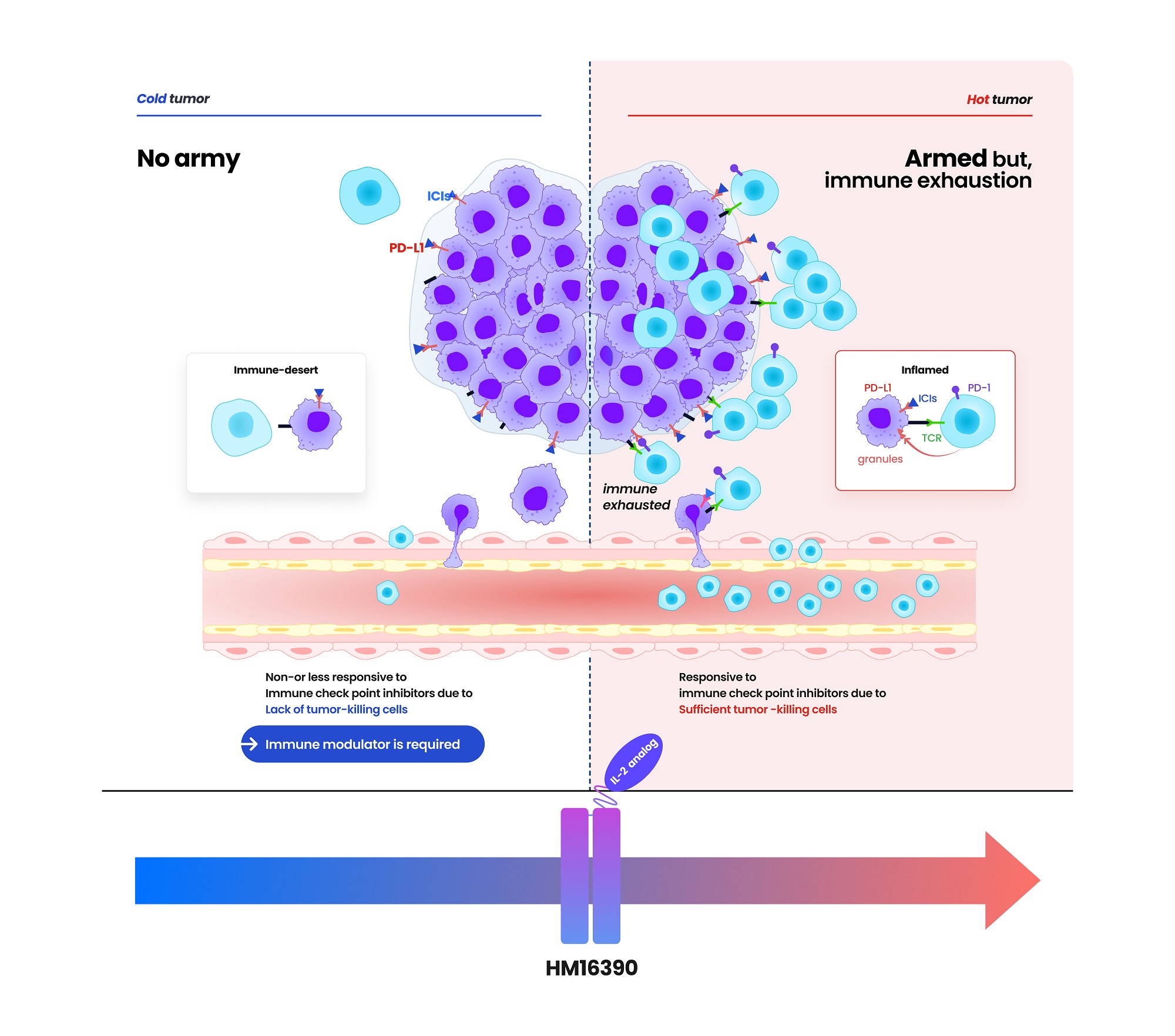
- Hanmi partners with MSD for next-gen IL-2 analog development
- by Cha, Jihyun May 20, 2025 05:58am
- Hanmi Pharmaceutical (CEO: Jae-Hyun Park) announced on the 19th that it has signed a clinical trial collaboration and supply agreement with U.S. Merck (MSD) to evaluate the combination therapy of its LAPS IL-2 analog 'HM16390' and MSD's anti-PD-1 immunotherapy 'Keytruda' (pembrolizumab). Hanmi Pharmaceutical will sponsor and oversee the Phase
- Company
- 'Oxlumo' for primary hyperoxaluria expected to be available
- by Eo, Yun-Ho May 19, 2025 05:56am
- The primary hyperoxaluria treatment, 'Oxlumo,' is expected to be commercialized in South Korea. According to sources, the Ministry of Food and Drug Safety (MFDS) reviewing Oxlumo (lumasiran) for approval. The MFDS granted 'Global Innovative products on Fast Track (GIFT)' designation to Oxlumo last year and orphan drug status in October
- Company
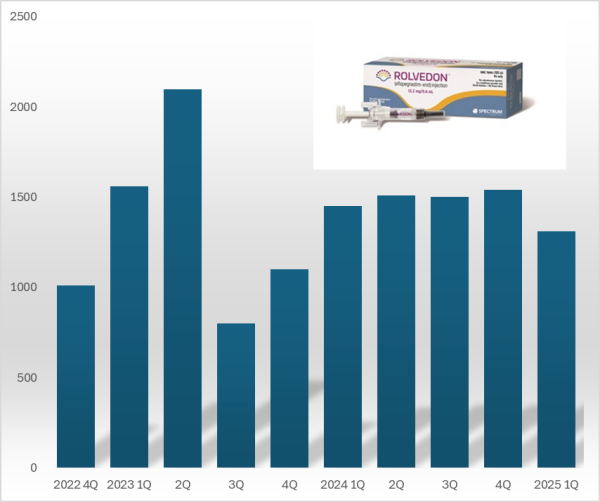
- Hanmi 'Rolvedon' reports US sales gain ₩18B in Q1
- by Chon, Seung-Hyun May 19, 2025 05:55am
- 'Rolvedon,' a treatment for neutropenia that Hanmi Pharmaceutical licensed out, continues to be popular in the U.S. market. Although the growth trend has stalled due to price reduction in the U.S., Rolvedon recorded over US$ 10 million in sales for six consecutive years. Rolvedon's cumulative sales amounted to KRW 200 billion in two years and si
- Company
- Ebglyss may be prescribed in general hospitals in Korea
- by Eo, Yun-Ho May 16, 2025 06:21am
- The new drug Ebglyss for atopic dermatitis may be prescribed in general hospitals in Korea. According to industry sources, Lilly Korea's interleukin (IL)-13 inhibitor Ebglyss (lebrikizumab) has passed the Drug Committees (DCs) of 9 medical institutions nationwide, including tertiary hospitals like Asan Medical Center and Sinchon Severance
- Company
- AZ 'Imfinzi' leads the paradigm shift in cholangiocarcinoma
- by Whang, byung-woo May 16, 2025 06:18am
- "Introduction of Imfinzi in cholangiocarcinoma treatment can be seen as a critical advance. That a new therapy offering the possibility of long-term survival has appeared after 12 years is highly encouraging." As new treatment options for cholangiocarcinoma are introduced, a paradigm shift is said to be brought to this area, which was previou
- Company
- Doctors ‘Reimb too slow for new drugs in Korea’
- by Eo, Yun-Ho May 15, 2025 06:23am
- Most doctors were found to believe that the speed of reimbursement for new drugs in Korea is too slow. The Korean Research-based Pharmaceutical Industry Association (KRPIA) released the results of a survey of 100 domestic medical professionals on the 14th. In January, the global polling agency Ipsos Research surveyed domestic clinical
- Company
- Arexvy opening the era of RSV vaccine
- by Whang, byung-woo May 15, 2025 06:22am
- As GSK launches the respiratory syncytial virus (RSV) vaccine Arexvy in South Korea, it will challenge the market on a full-scale. Arexvy is already expanding its market dominance in the global market with its strength as the first RSV vaccine. The company will likely focus on expanding vaccine awareness as it opens the RSV vaccine market for