- Company
- Eylea biosimilar by SCD applies for Phase 3 global IND
- by Lee, Seok-Jun Jan 29, 2020 06:25am
- Samchundang Pharm (SCD) plans to have Phase 3 global clinical trial protocol approved for Eylea’s biosimilar ‘SCD411’ within the first quarter of the year. The protocol approval would be reviewed for 90 days at longest, and green light the company to start the Phase 3 trial by the coming second quarter. The multiregional study is planned
- Company
- General hospitals clear Norvasc for pediatric use
- by Eo, Yun-Ho Jan 28, 2020 11:18am
- More general hospitals are reportedly listing Norvasc on their drug codes for pediatric treatment. Pharmaceutical industry on Jan. 28 said respective Drug Committees in Seoul National University Hospital, Severance Hospital and Korea University Guro Hospital cleared Norvasc (Amlodipine) 2.5 mg for prescription from the release in last ye
- Company
- Repatha’s options for ultra high risk patients extended
- by Eo, Yun-Ho Jan 28, 2020 06:13am
- Since statins, the coverage of the PCK9 inhibitor Repatha, an option for managing dyslipidemia, is expected to increase utilization. Amgen Korea(the CEO Noh) held a press conference at the Westin Chosun Hotel in Seoul on the 22nd to commemorate the reinforcement of health insurance benefits for Repatha’s atherosclerotic cardiovascular dis
- Company
- Last year, Tamiflu sales fell by half
- by Jung, Hye-Jin Jan 28, 2020 06:13am
- Last year, the flu treatment Tamiflu market dropped sharply. The size of the market has shrunk as the number of flu patients has decreased significantly from 2018. The share of generics in the market has been expanding. Outpatient prescription performance of Oseltamivir were down &8361;17.2 billion, down 50.4% from last year, according to UB
- Company
- Yuhan talks plans after lazertinib L/O at JPM Conference
- by Jung, Hye-Jin Jan 28, 2020 06:12am
- Yuhan announced on Jan. 22, the company has participated in J.P. Morgan Healthcare Conference 2020 to talk about plans following the out-licensing lazertinib and to hold R&D recruiting event. Since 2018, Yuhan has signed 3.5 trillion won worth of out-licensing deals including lazertinib. And at the event in the U.S., the company represen
- Company
- Sanofi firing employee for playing golf is unfair dismissal
- by Eo, Yun-Ho Jan 23, 2020 06:16am
- A government organization has decided a salesperson fired by Sanofi Pasteur for playing golf with his colleagues during their working time was unfair dismissal. Following the decision made by Chungnam Regional Labor Relations Commission in 2019, National Labor Relations Commission (NLRC) has accepted “Manager A”’s request for unfair
- Company
- Lilly Korea, certified as a family-friendly company
- by Eo, Yun-Ho Jan 23, 2020 06:15am
- Lilly Korea (CEO Alberto Riva) said it has succeeded for receiving the family-friendly company certification, which is given to companies that run family-friendly models, for 10 consecutive years by the Ministry of Gender Equality and Family. The Family-Friendly Certification System, through the screening of the Ministry of Gender Equality
- Company
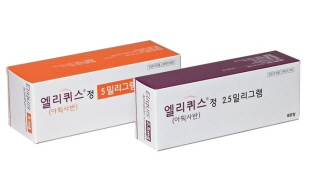
- Eliquis flying out to space and saving an astronaut?
- by Eo, Yun-Ho Jan 22, 2020 10:47pm
- Apparently, anticoagulant Eliquis (Apixaban) has been delivered to outer space. This was the first outer space deep vein thrombosis (DVT) treatment and prescription in the history. The details were revealed when an article titled ‘Venous Thrombosis during Spaceflight’ was published in New England Journal of Medicine (NEJM).
- Company
- BMS-Celgene to maintain independent offices this year
- by Eo, Yun-Ho Jan 22, 2020 06:29am
- Bristol-Myers Squibb (BMS) and Celgene have agreed to maintain the independent administrative system until the end of the year. According to pharmaceutical industry sources, BMS Korea Pharmaceutical and Celgene Korea have recently convened a town hall meeting to discuss the post-merge operational plan for the Korean offshoots, and have d
- Company
- Despite No Japan movement, Japanese companies performed well
- by An, Kyung-Jin Jan 22, 2020 06:28am
- In the prescription drug market, there was no aftermath of a Japanese boycott. Since July, when the Japanese government began to regulate exports, outpatient prescriptions by Japanese pharmaceutical companies have increased. The high severity of disease and the proportion of original drugs have unaffected by the boycott. According to UB