- Company
- New AML drug Vyxeos can be prescribed at Big 5 hospitals
- by Eo, Yun-Ho Nov 1, 2024 05:51am
- The new acute myeloid leukemia drug ‘Vyxeos’ may now be prescribed at general hospitals in Korea. According to industry sources, Vyxeos (daunorubicin+ cytarabine), a treatment for adults with acute myeloid leukemia AML, has passed the drug committees (DCs) of the ‘Big 5’ tertiary hospitals in Korea including the Samsung Medical Center
- Company
- Novartis’s operating income grew 123% in Q3 with Entresto
- by Son, Hyung Min Nov 1, 2024 05:50am
- The Swiss global pharmaceutical giant Novartis' sales increased slightly compared to the previous year. Novartis showed even sales growth in various therapeutic areas, including cardiovascular, anticancer, and immunosuppressive agents. According to industry sources, Novartis reported a revenue of USD 12.823 billion in Q3 last year, up 10% YoY
- Company
- Atopic dermatitis drug Ebglyss to enter Korea next year
- by Whang, byung-woo Oct 31, 2024 05:55am
- The atopic dermatitis drug Ebglyss (lebrikizumab), which is set to soon be released in Korea, has added competitivity by securing rationale for switching between biologics. The company plans to receive reimbursement after launching the drug without reimbursement early next year. At the recent Fall Clinical Dermatology conference that
- Company
- Oral ALS drug 'Radicava' likely to land in KOR
- by Eo, Yun-Ho Oct 31, 2024 05:55am
- 'Radicava,' an oral drug for the treatment of amyotrophic lateral sclerosis (ALS), is expected to be commercialized in South Korea. Sources said that Mitsubishi Tanabe Pharma Korea's Radicava (edaravone) is under consideration for domestic approval. The drug is a treatment for ALS, formerly known as Lou Gehrig's disease. Radicava is
- Company
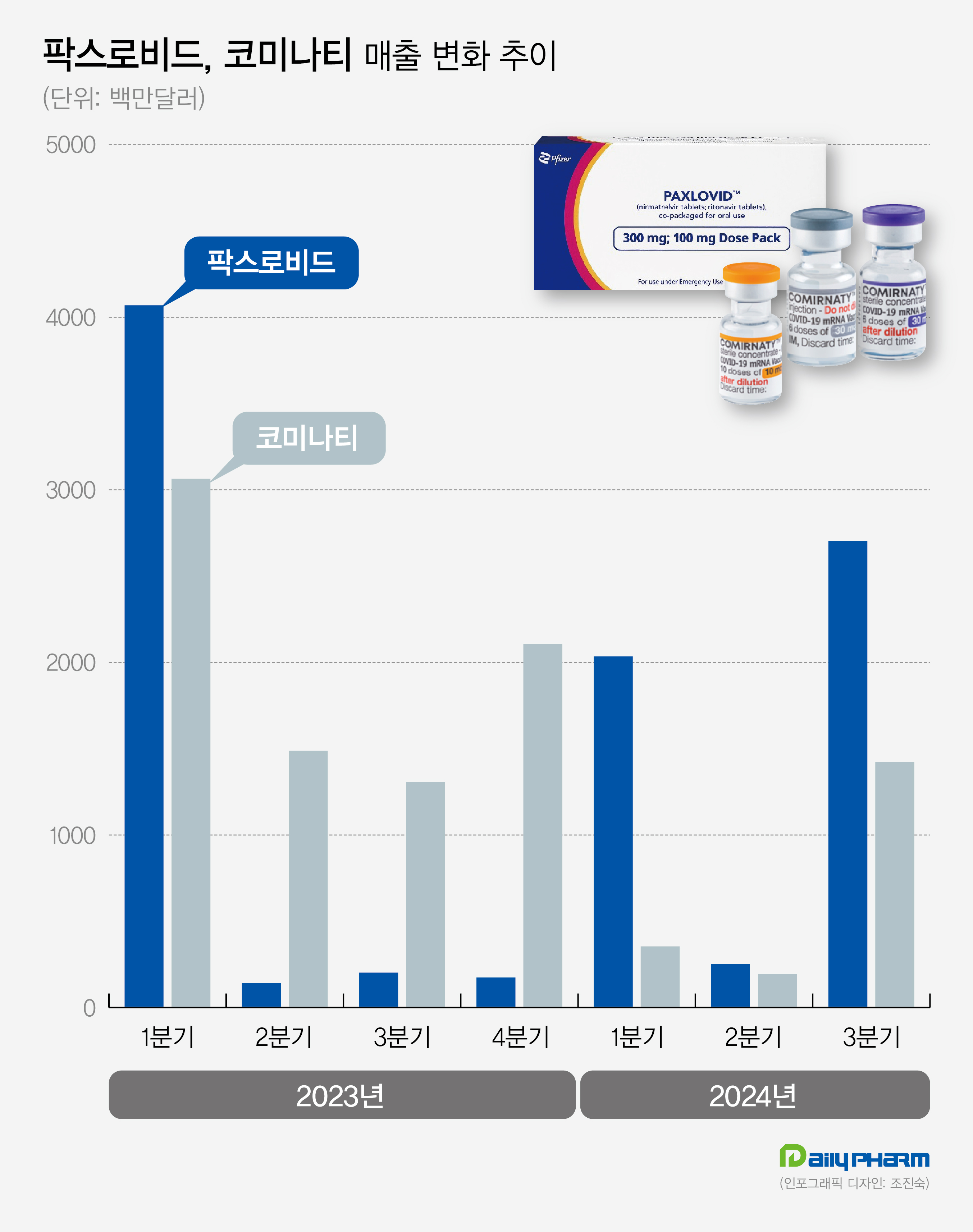
- Pfizer's Q3 sales 31%↑amid increased demand for COVID-19
- by Son, Hyung Min Oct 31, 2024 05:55am
- The global pharmaceutical company Pfizer's sales are increasing compared to the previous year. Pfizer's growth has been driven by the sales of COVID-19 vaccines and treatments such as Paxlovid and Comirnaty. According to industry sources on October 30, Pfizer's sales in Q3 recorded US$17.72 billion (about KRW 24.48 trillion), up 31.2% year-ov
- Company
- NIP inclusion of HPV vaccines on the horizon
- by Whang, byung-woo Oct 31, 2024 05:55am
- Amid high interest in the expansion of the National Immunization Program (NIP) to include HPV vaccines, the possibility of its inclusion in 2026 has arisen in Korea. Although the agenda is yet in the planning stage, attention is being paid to whether it can gain substance in line with the NA budget deliberation discussions next month.
- Company
- Difficulty expanding indications for antibody-drug conjugate
- by Moon, sung-ho Oct 30, 2024 05:54am
- Drug candidates that received much expectation as next-generation antibody-drug conjugates (ADCs) based on the success of Enhertu are on a struggling path to reimbursement in Korea. &160; Although the companies aimed to expand the scope of coverage of their drugs based on their effectiveness in specific cancer types, the expectations damped
- Company
- Dupixent sales skyrocket with 'expanded indications'
- by Son, Hyung Min Oct 30, 2024 05:54am
- Sales for 'Dupixent,' a biological agent developed by Sanofi, continue to skyrocket. Net sales for Dupixent from Q1 to Q3 of 2024 reached 10 billion euros. The analysis suggests that Dupixent's added indications to treat various immune diseases, including atopic dermatitis, asthma, esophagitis, and chronic obstructive pulmonary disease (COPD), h
- Company
- "Early intervention" needed for treating multiple myeloma
- by Whang, byung-woo Oct 29, 2024 05:49am
- The survival rate of patients with multiple myeloma increased following new drug development. However, concerns have been raised that South Korea's survival rate is still far from that of advanced countries. Expert opinions indicate that the Korean medical treatment field changes with new drug approvals and reimbursement listings, yet patien
- Company
- Will the third time be the charm for Mylotarg?
- by Eo, Yun-Ho Oct 29, 2024 05:49am
- The industry’s attention is focused on whether the insurance reimbursement discussions for the acute myeloid leukemia drug ‘Mylotarg’ will make progress this time. According to the industry sources, Pfizer Korea’s acute myeloid leukemia (AML) drug ‘Mylotarg (gemtuzumab ozogamicin)’ is set to be submitted to the Health Insurance Revi