- Policy
- Gov’t raises service fee for difficult surgeries
- by Lee, Tak-Sun Aug 14, 2024 05:51am
- The government is promoting a plan to completely reform the unbalanced structure of Korea’s fee-for-service system. For this, the government is reviewing whether to raise the compensation level for severe surgeries and reforming the relative value system and conversion index used to calculate medical service fees. Kyung-sil Jeong, Hea
- Policy
- Coinsurance rate for chronic diseases reduced from 30%→20%
- by Lee, Hye-Kyung Aug 14, 2024 05:51am
- 1 The coinsurance rate for outpatient treatment at neighborhood clinics for chronic diseases such as hypertension and diabetes will be reduced by 10%, from 30% to 20%. The Ministry of Health and Welfare announced on the 13th that a partial amendment to the ‘Enforcement Decree of the National Health Insurance Act’ was approved at a cabinet
- Product
- Concerns arise about securing COVID-19 treatment shortages
- by Kang, Hye-Kyung Aug 14, 2024 05:51am
- COVID-19 is spreading due to the circulating KP.3 variant. We have not learned lessons from the previous spread of the Omicron variant. As demand for test kits surges, there are no remaining stocks at online pharmacies. Even test kits with an expiration date within the end of October are no longer available. Due to shortages of oral medicine
- Company
- 'Xtandi and Erleada' compete in the prostate cancer market
- by Hwang, Byung-woo Aug 14, 2024 05:51am
- The market for ARTA treatment used to treat prostate cancer has been dominated by Xtandi (enzalutamide). As prescription sales of Erleada (apalutamide) rise, the market is shifting. Astellas Pharma's Xtandi has increased its competitiveness with its indications, Janssen has started to face competition by expanding its portfolio, including Er
- Opinion
- [Reporter's View] Delays in medication switching for eczema
- by Eo, Yun-Ho Aug 13, 2024 05:48am
- When patients switch from their current medication to a different medication, the switched products are not covered by insurance reimbursement. This non-reimbursed status of medication switching has been a long-standing issue in South Korea. The field most impacted is atopic dermatitis. Treatment options had been limited for atopic derma
- Company
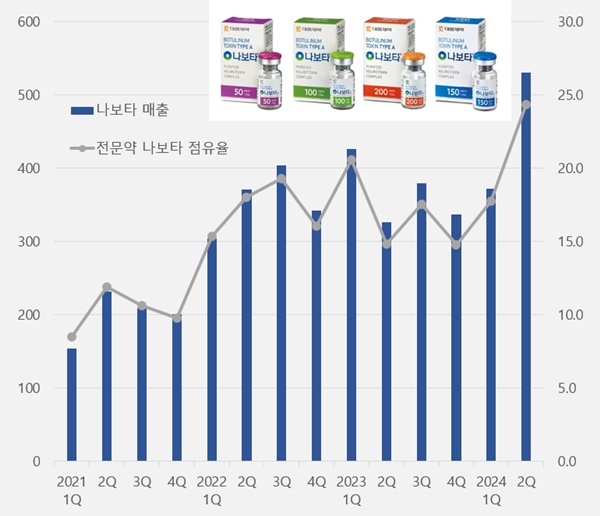
- Nabota accounts for 24% of Daewoong’s ETC sales
- by Chon, Seung-Hyun Aug 13, 2024 05:47am
- Daewoong Pharmaceutical's botulinum toxin 'Nabota' continued its upward sales trend. The company's quarterly revenue exceeded KRW 50 billion for the first time, led by the company’s growth in overseas markets. Nabota’s share of Daewoong's specialty drug sales reached nearly 25 percent, driving the company's performance. According to Daewoon
- Company
- 'High immunity vs. high dose’ flu vaccine for older adults
- by Hwang, Byung-woo Aug 13, 2024 05:47am
- Equipped with their respective flu vaccines specialized for people aged 65 and older, Sanofi and CSL Seqirus are seeking to expand their market share in Korea’s influenza (flu) vaccine market, which is driven by vaccines registered in the National Immunization Program (NIP). CSL Seqirus was the first to enter this market last year, followed
- Company
- Hanmi’s Rolvedon posts sales of KRW 20.6 billion in Q2
- by Son, Hyung-Min Aug 13, 2024 05:47am
- Sales of Rolvedon (Korean brand name: Rolontis), a new anti-cancer drug developed by Hanmi Pharmaceutical, are showing signs of recovery in the U.S. market. Hanmi Pharmaceutical's U.S. partner Assertio plans to increase Rolvedon’s market share through new clinical trials that could ensure the drug’s advantage in convenience of administration.
- Company
- JAK inhibitors market size rose 54%↑over the year
- by Kim, Jin-Gu Aug 12, 2024 05:55am
- The market for Janus Kinase (JAK) inhibitors, oral medicines used to treat autoimmune diseases, is growing rapidly following the approval of major medicines for expanded indications. In the first half of the year, the market for JAK inhibitors reached KRW 27.5 billion in outpatient sales, up 54% year over year (YoY). Abbvie's 'Rinvoq
- Company
- Columvi can be prescribed at general hospitals in KOR
- by Eo, Yun-Ho Aug 12, 2024 05:55am
- ‘Columvi,' the first bispecific antibody treatment option for lymphoma, may be prescribed at general hospitals in Korea. According to industry sources on the 9th, Roche Korea's CD20-CD3 bispecific antibody for diffuse large B-cell lymphoma (DLBCL) Columvi (glofitamab) passed Samsung Medical Center’s drug committee review. However, Co