- Company
- Lilly seeks Mounjaro’s reimbursement for diabetes in Korea
- by Eo, Yun-Ho Aug 22, 2025 06:08am
- ‘Mounjaro,’ which is causing a roar in the field of obesity treatment, is seeking inclusion in insurance reimbursement for its diabetes indication. According to Dailpharm coverage, Eli Lilly Korea has submitted a reimbursement application for its Mounjaro (tirzepatide), a dual GIP/GLP-1 receptor agonist, and is proceeding with the neces
- Policy
- Blockbuster drugs, Atozet·Rosuzet, get price cuts
- by Lee, Tak-Sun Aug 22, 2025 06:07am
- It has been reported that prices for blockbuster drugs, including the hyperlipidemia combination therapies Atozet (Organon) and Rosuzet (Hanmi Pharmaceutical), are expected to be reduced due to increased usage. These hyperlipidemia combination therapies are frequently subject to annual volume-based drug price negotiations (PVA). Addit
- Policy
- ALS drug Qalsody is approved in Korea with a condition
- by Lee, Hye-Kyung Aug 22, 2025 06:07am
- The ALS treatment ‘Qalsody (tofersen)’ has been approved under the condition that the results of its therapeutic confirmatory clinical trial be submitted later. According to the advisory council’s review results regarding the safety and efficacy of Qalsody, which was released by the Ministry of Food and Drug Safety on the 20th, the cou
- Opinion
- [Reporter's View] Double standard against natural medicines
- by Kim, Jin-Gu Aug 22, 2025 06:06am
- The government announced the '4th New Natural Product Drug Development Promotion Plan' in May 2024. The plan outlines a joint effort by seven ministries, including the Ministry of Health and Welfare, the Ministry of Science and ICT, Ministry of Trade, Industry and Energy, Ministry of Environment, Ministry of Oceans and Fisheries, Rural Devel
- Company
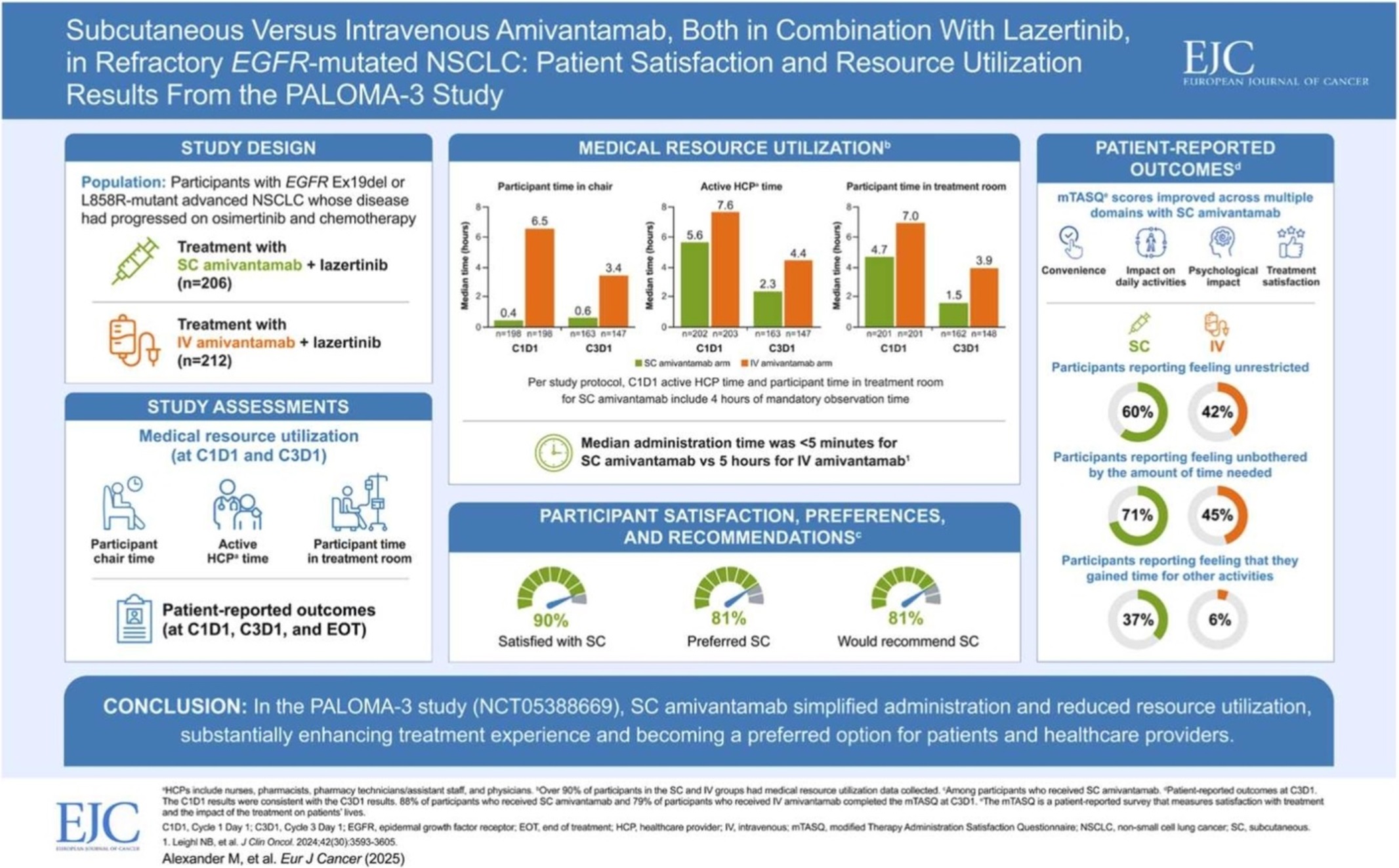
- Leclaza at global crossroads 1 year into FDA approval
- by Moon, sung-ho Aug 22, 2025 06:06am
- One year has passed since Leclaza (lazertinib) received FDA approval in combination with Johnson & Johnson’s Rybrevant (amivantamab). Expanding its influence beyond the United States to Europe and Asia, it has emerged as a global treatment option both in Korea and abroad. In the first half of this year, it recorded an overall survival rate (
- Product
- Mounjaro arrives at pharmacies..₩310,000–350,000
- by Kang, Hye-Kyung Aug 21, 2025 06:07am
- Mounjaro (tirzepatide), which had been attracting considerable attention even before its arrival in Korea, has now been made available to pharmacies. With prescriptions also beginning on the 20th, pharmacies are bustling with activity. According to local pharmacies, two dosage forms, 2.5mg and 5mg, have begun arriving at pharmacies through wh
- Company
- Nubeqa gains flexibility with indication expansion
- by Whang, byung-woo Aug 21, 2025 06:06am
- The influence of Nubeqa (darolutamide) is rising in the market with the company expanding its indication as a treatment for metastatic hormone-sensitive prostate cancer (mHSPC). Experts believe that the drug may settle as a flexible treatment option in Korea’s market as it has broadened its path as a personalized treatment. With the approval
- Company
- Reimb of polycythemia vera drug 'BESREMi' likely in Sept
- by Eo, Yun-Ho Aug 21, 2025 06:06am
- The polycythemia vera treatment 'BESREMi' is expected to be listed on the national health insurance list. The National Health Insurance Service (NHIS) and PharmaEssentia Korea have recently reached a final agreement on the drug price negotiation for BESREMi (ropeginterferon alfa-2b). As a result, if it passes the Health Insurance Policy Re
- Company
- Viatris signs exclusive sales and distribution deal for Brid
- by Whang, byung-woo Aug 21, 2025 06:05am
- Viatris Korea announced on the 20th that it has signed an exclusive domestic promotion and distribution agreement for the general anesthesia reversal agent Bridion (Sugammadex) through a strategic partnership with MSD Korea. Under the agreement, Viatris Korea officially took over the domestic promotion and distribution of Bridion as of th
- Company
- Generics challenge the patent of mkt leading 'Rinvoq'
- by Kim, Jin-Gu Aug 21, 2025 06:05am
- The patent challenges by generics targeting AbbVie's Janus kinase (JAK) inhibitor' Rinvoq (upadacitinib)' have begun. The pharmaceutical industry anticipates that patent challenges will further expand as Rinvoq strengthens its monopolistic position in the JAK inhibitor market, which is valued at approximately KRW 62 billion annually.