- Leclaza at global crossroads 1 year into FDA approval
- by Moon, sung-ho | translator Alice Kang | Aug 22, 2025 06:06am
Expanding its influence beyond the United States to Europe and Asia, it has emerged as a global treatment option both in Korea and abroad. In the first half of this year, it recorded an overall survival rate (OS) exceeding 50 months, emerging as a global standard of care for non-small cell lung cancer and contributing to a major shift in the treatment paradigm.
According to industry sources on the 18th, the FDA approved the use of Rybrevant in combination with Leclaza (US brand name: Lazcluze) as a first-line treatment for adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) with EGFR exon 19 deletion or exon 21 L858R substitution mutation, or adult patients with locally advanced or metastatic non-small cell lung cancer (NSCLC).
The combination was also approved in Europe in December of the same year, followed by the UK (March), Japan (March), and Canada (March) in the first quarter of this year, and China (July) in the second quarter.
In May, prescriptions began in Japan, indicating the combination is gaining momentum in its expansion into the global market.
Among the countries that have approved the combination, the combination’s performance in China is particularly noteworthy. With approximately 1 million new lung cancer diagnoses each year, lung cancer is the No.1 cancer in terms of incidence and mortality in China.
This accounts for more than one-third of the 2.5 million new lung cancer cases worldwide. In addition, approximately 85% of lung cancer patients in China have non-small cell lung cancer (EGFR accounts for 40% of non-small cell lung cancer).
Furthermore, the OS results of the Phase III (MARIPOSA) study presented at the European Lung Cancer Congress (ELCC 2025) held in Paris, France, in March this year have further accelerated the growth momentum.
According to the Phase III MARIPOSA study, the combination therapy arm that received Rybrevant and Leclaza saw a 25% reduction in risk of death compared to the Tagrisso (osimertinib, AstraZeneca) arm (HR=0.75, 95% CI: 0.61–0.92, P<0.005). The median overall survival (mOS) in the combination therapy arm has not been reached and was analyzed as NE (95% CI: 42.9–NE), while the Tagrisso arm’s mOS was 36.7 months (95% CI: 33.4–41.0).
This suggests that the use of the combination therapy can add a survival period of over one year when compared to Tagrisso monotherapy.
Based on these results, Yuhan Corp, which successfully licensed out Leclaza, is also achieving financial results in line with the global expansion of Leclaza. The total scale of the licensing out contract for Leclaza that Yuhan Corp signed with Johnson & Johnson in 2018 (including an upfront payment of USD 50 million) was USD 1.255 billion, and following a partial revision of the contract in September last year, the total scale was revised to USD 950 million. The milestone payment alone amounts to USD 900 million.
Yuhan Corp has already received USD 175 million from the amount. Specifically, it received payments for: ▲Combination therapy development (April 2020, USD 35 million) , ▲Initiation of Phase III clinical trials as combination therapy (November 2020, USD 65 million), ▲Initiation of its commercialization in the U.S. (September 2024, USD 60 million), ▲Japan commercialization (May 2025, USD 15 million). The remaining milestone payments total at USD 725 million.
Additionally, the company will receive “royalties on net sales” from global sales of Leclaza separately from the milestone payments. The amount is reported to be over 10% of net sales.J&J currently discloses combined sales of Rybrevant and Leclaza in its quarterly earnings reports. Global sales for the second quarter of this year were USD 179 million, representing a 159% increase from the same period last year and a 27% increase from the previous quarter. Combined sales for the first half of the year, including the first quarter, amounted to USD 320 million.
The pharmaceutical industry believes that the successful expansion of Lecclaza's position in the global market will depend on whether Rybrevant SC, which is currently under review, will be approved by the FDA.
Although inclusion in the NCCN guidelines as the first-line treatment is important, the approval of Rybrevant SC is the biggest variable for the combination’s rapid adoption in clinical practice.
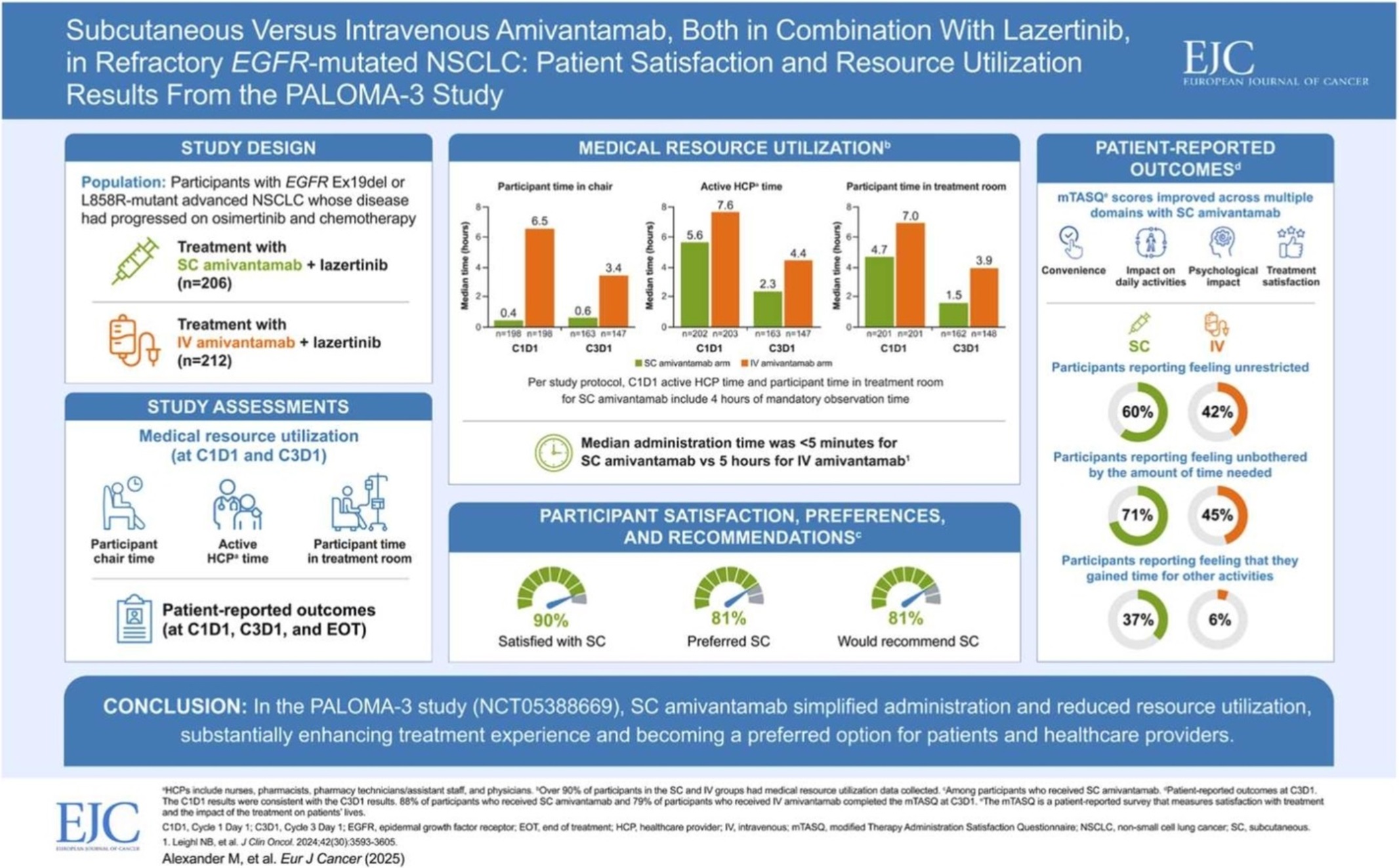
J&J is confident that it will receive FDA approval for Rybrevant SC in the second half of this year based on the PALOMA-3 study, which served as the basis for the application. For reference, the FDA sent a complete response letter (CRL) to J&J regarding Rybrevant SC in December last year.
Jennifer Taubert, Executive Vice President and Worldwide Chairman of Innovative Medicine at Johnson & Johnson, stated, “We have already responded to the FDA's CRL. There were no requests for additional clinical trials or clinical data submissions -- only one or two manufacturing-related questions. We have completed our responses and are hoping to receive approval in the second half of the year.”
Another variable is the OS results of the FLAURA2 Phase III trial on the two-drug regimen of Tagrisso and chemotherapy, which AstraZeneca plans to present at the World Conference on Lung Cancer (WCLC 2025) in September.
The FLAURA2 study enrolled 557 adult patients with EGFR-mutated locally advanced (stage 2B–3C) or metastatic (stage 4) non-small cell lung cancer, and randomized them to receive either Tagrisso 80 mg alone (278 patients) or Tagrisso 80 mg plus Alimta (pemetrexed) plus cisplatin/carboplatin (279 patients).
According to results published in 2023, the primary endpoint, investigator-assessed progression-free survival (PFS), was 25.5 months in the Tagrisso+chemotherapy combination group, extending the median PFS by 8.8 months compared to Tagrisso alone (16.7 months).
An industry insider commented, “When reviewing the FLAURA2 study presented at ESMO Asia last year, of which 60% were Asian patients, the median overall survival (OS) was 40.5 months and 38.3 months, respectively, showing a 2-month improvement as a two-drug regimen. This suggests that a relatively larger OS difference may be observed in non-Asian patients.”
With AstraZeneca announcing statistically significant improvements in OS, a secondary endpoint, attention is now focused on the full OS data from the FLAURA2 study that will be presented at WCLC 2025.
The combination therapy of Rybrevant and Lecclaza, which is indicated as the same line of treatment, has yielded OS data of over 50 months, allowing a direct comparison. The focus is on how much greater OS benefit the Tagrisso-chemotherapy combination therapy can provide relative to the Rybrevant-chemotherapy combination therapy, especially when chemotherapy is administered earlier in the treatment regimen.
This can be a bothersome development for the Rybrevant-Leclaza combination therapy, which is competing for the global preferred regimen position.
The global expansion and scale of achievements for Leclaza could vary significantly depending on the outcome of the Tagrisso-chemotherapy combination therapy OS results and the Rybrevant SC approval.
A representative from Yuhan Corp explained, “Currently, Rybrevant SC has been approved for prescription in Europe, and we are awaiting additional global approvals, including in the United States. The SC formulation reduces the administration time from approximately 6 hours with the existing intravenous (IV) formulation to about 5 minutes, offering various benefits such as improved convenience in administration.”
The representative further noted, “We are also accelerating clinical trials for adverse effect management. J&J presented interim results from a study on reducing the adverse effects of the combination therapy at ASCO 2025. Through preventive skin management, we have reduced adverse events by approximately one-third, and clinical trials are ongoing to further verify its safety in addition to its effect.”
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.
- [Op-Ed] Patients, no time left for 'new drug comb therapies'
- Special Contribution | Eo, Yun-Ho









