- Company
- SK Life Science’s sales rise tenfold in 5 years in the US
- by Chon, Seung-Hyun Mar 20, 2024 05:44am
- SK Biopharmaceuticals' U.S.-based subsidiary posted sales of nearly KRW 500 billion last year. The sales of its epilepsy drug cenobamate increased in the U.S., increasing the company’s sales by over tenfold in 5 years. According to the Financial Supervisory Service on Tuesday, SK Life Science’s sales totaled KRW 49.9 billion last year, up
- Company
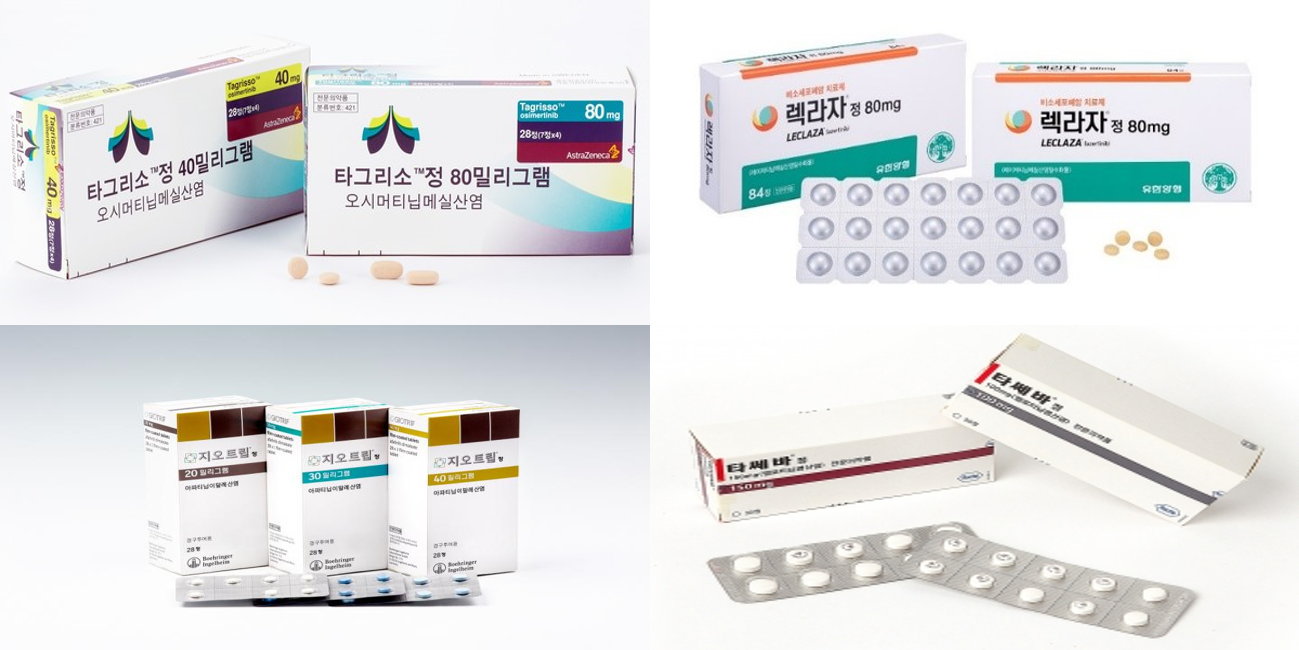
- Sales of Tagrisso stay strong, Leclaza rises in lung cancer
- by Mar 20, 2024 05:44am
- In the EGFR-positive non-small cell lung cancer (NSCLC) treatment market, Leclaza’s sales growth stood out amid strong sales of Tagrisso. Tagrisso and Leclaza are both third-generation targeted therapies, and the two were approved as a first-line treatment for lung cancer this year and were given the green light to expand sales. Among firs
- Company
- Professor Yoo discovers specific protein expression in HCC
- by Lee, Tak-Sun Mar 19, 2024 05:45am
- On the 15th, CMIC Korea, a contract research organization (CRO), announced that the results of the RENOBATE (IIT, Phase II) trial led by Professor Changhoon Yoo (Department of Oncology, Asan Medical Center) were recently published in the international academic journal, Nature Medicine (IF=82.9). This is the first time the results of a clin
- Company
- Celltrion rolls out Zymfentra in the U.S.…its 1st new drug
- by Chon, Seung-Hyun Mar 19, 2024 05:44am
- On the 18th, Celltrion announced that it had launched Zymfentra, a subcutaneous (SC) formulation of infliximab, in the United States. Zymfentra is a subcutaneous injection formulation of Celltrion's infliximab. The company received approval from the U.S. Food and Drug Administration last year for its use as a treatment for patients with u
- Opinion
- [Reporter’s View] Let Enhertu’s case guide new listings
- by Eo, Yun-Ho Mar 19, 2024 05:44am
- The reimbursement listing of the advanced ADC anti-cancer drug Enhertu is imminent. The company completed drug pricing negotiations with the National Health Insurance Service at a ‘superfast’ speed when considering the slow progress it had made during previous procedures. At this pace, Enhertu is likely to be reimbursed in April this year.
- Policy
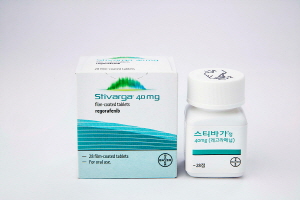
- Liver cancer therapy, ‘Stivarga’ set for 2nd RSA renewal
- by Lee, Tak-Sun Mar 19, 2024 05:44am
- It has been reported that a risk sharing agreement (RSA) has been renewed for the second time for Stivarga (regorafenib; Bayer Korea), a second-line treatment for liver cancer. The news comes two months before the reimbursable RSA contract for Stivarga is set to expire on May 31st. According to industry sources on the 18th, the Nationa
- Company
- New CKD drug Kerendia is reimbursed in Korea
- by Son, Hyung-Min Mar 18, 2024 05:50am
- Whether the reimbursement coverage of Bayer’s Kerendia will help address the unmet needs of patients with chronic kidney disease with type 2 diabetes, is gaining attention. Until now, blood pressure medications and diabetes drugs have been used to treat chronic kidney disease, but the introduction of Kerendia has expanded the treatment opti
- Company
- NMOSD drug Enspryng can be prescribed at general hospitals
- by Eo, Yun-Ho Mar 18, 2024 05:50am
- Enspryng, a new drug for neuromyelitis optica spectrum disorder (NMOSD) is landing in general hospitals in Korea. According to industry sources, Roche Korea's Neuromyelitis Optica Spectrum Disorder (NMOSD) drug Enspryng (satralizumab) has passed the drug committees (DCs) of tertiary hospitals including Seoul National University Hospital,
- Policy
- Sotyktu, Adtralza set for reimbursement listing
- by Lee, Tak-Sun Mar 18, 2024 05:49am
- Two drugs, plaque psoriasis drug 'Sotyktu (deucravacitinib)' and atopic dermatitis drug 'Adtralza (tralokinumab)' have completed the negotiation with the National Health Insurance Service (NHIS) and may soon be listed for reimbursement. Additionally, the upper price limit of Soliris (eculizumab) is expected to be reduced following the e
- Opinion
- [Reporter’s View] 'Tylenol,' no longer produced,
- by Lee, Hye-Kyung Mar 18, 2024 05:49am
- Twelve years have passed since the government desginated over-the-counter emergency medicines. On November 15, 2012, the government implemented 13 over-the-counter (OTC) emergency medicines, including antipyretic analgesics, gastrointestinal medicine, and patches. When designating 13 OTC emergency medicines, the government based its eval