- Company
- COVID-19 vaccination system has been included in the NIP
- by Whang, byung-woo Aug 6, 2025 06:09am
- As COVID-19 vaccination has been included in the National Immunization Program (NIP), changes to the operational system is anticipated. Previously, government provided COVID-19 immunization service for high-risk groups. Whereas the previous service had been temporary, the current vaccination system is expected to become more established. T
- Company
- Organon-KSCRH implements Implanon NTX Certificate Program
- by Whang, byung-woo Aug 5, 2025 06:08am
- The Implanon NXT Certificate Program, jointly implemented by Organon Korea and the Korean Society of Contraception and Reproductive Health, is yielding positive results. Organon Korea announced on the 4th that 172 medical professionals have received certification through the ‘Implanon NTX Certificate Program,’ which has been in operatio
- Company
- Generic companies challenge Xtandi’s patent in KOR
- by Kim, Jin-Gu Aug 5, 2025 06:08am
- Astellas' prostate cancer treatment ‘Xtandi (enzalutamide)’ has become the target of a patent challenge by a generic drug company. According to industry sources on the 4th, Alvogen Korea recently filed a request for invalidation trial (passive scope confirmation trial) of the composition patent for Xtandi against Astellas. This is the
- Policy
- Faslodex’s price stays as is…supply instability concerns
- by Lee, Tak-Sun Aug 5, 2025 06:08am
- The breast cancer drug Faslodex (fulvestrant, AZ Korea), was facing a price cut due to the expiry of its premium pricing period, but this was prevented by the pharmaceutical company's request for a stay of execution. On the 31st of last month, the MOHW announced that the court had temporarily suspended the execution of the disposition to
- Company
- Inclusion of Prevenar 20 into NIP for children starting Oct
- by Whang, byung-woo Aug 5, 2025 06:08am
- As Prevenar 20 has been confirmed to be included in the National Immunization Program (NIP) in October, a fierce market competition is anticipated. The implementation has been delayed from its initial expected schedule in Q3. However, it is anticipated to shift the market for pneumococcal conjugate vaccines with the emergence of the newest
- Company
- Galafold reimbursed as first-line drug for Fabry disease
- by Nho, Byung Chul Aug 4, 2025 05:54am
- Reimbursement for Handok's Fabry disease treatment, Galafold (migalastat), will be expanded to cover its use as a first-line treatment starting on the first of this month. Previously, Galafold was covered only for patients aged 16 and older who had been administered enzyme replacement therapy (ERT) intravenously for at least 12 months.
- Company
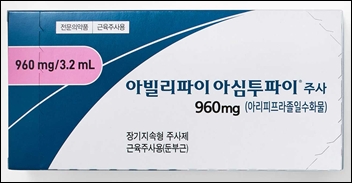
- Otsuka launches once-every-2-months inj 'Abilify Asimtufii'
- by Whang, byung-woo Aug 4, 2025 05:54am
- Otsuka Pharmaceutical Korea announced on August 1 that it will launch 'Abilify Asimtufii Inj (aripiprazole monohydrate),' starting in August, as the reimbursement coverage by the National Health Insurance has been applied. 'Abilify Asimtufii Inj' is an extended-release formulation administered once every two months. It was approved on Feb
- Policy
- MFDS clarifies support for advanced biopharmaceuticals
- by Lee, Hye-Kyung Aug 4, 2025 05:53am
- With the Ministry of Food and Drug Safety raising the approval and review fees for advanced biopharmaceuticals by up to KRW 410 million from January 1 this year, it has clarified the criteria for those eligible for fee reductions. In addition, detailed administrative disposition standards have been set for cases where human cell managers and
- Company
- Integrated CKM approach, the new paradigm for CKD treatment
- by Whang, byung-woo Aug 4, 2025 05:53am
- “In the past, we relied solely on single agents such as ACE inhibitors or ARBs to treat chronic kidney disease, but now an integrated approach that simultaneously manages cardiovascular, renal, and metabolic conditions is rising as the option. In this context, the results of the CONFIDENCE study, a recent study on the early combined use of SGLT
- Company
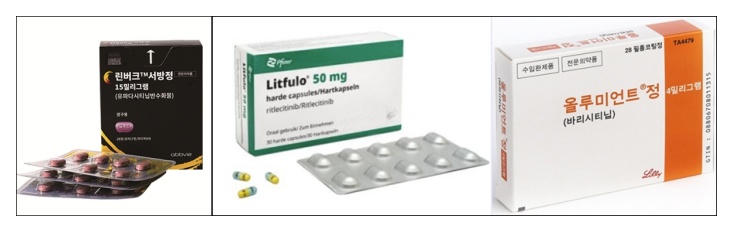
- Rinvoq joins the competition in the alopecia areata trt. mkt
- by Whang, byung-woo Aug 4, 2025 05:53am
- AbbVie's JAK inhibitor Rinvoq (upadacitinib) is expected to enter the market competition following successful clinical trials for alopecia areata. AbbVie recently announced positive topline results from the No.2 study of the Phase 3 clinical program (UP-AA program) for Rinvoq in adult and adolescent patients with severe alopecia areata.