- COVID-19 vaccination system has been included in the NIP
- by Whang, byung-woo | translator Hong, Ji Yeon | Aug 6, 2025 06:09am
Previously, government provided COVID-19 immunization service for high-risk groups. Whereas the previous service had been temporary, the current vaccination system is expected to become more established.
The Korea Disease Control and Prevention Agency (KDCA) announced that it will procure a total of 5.3 million doses of vaccine for the 2025-2026 season's COVID-19 NIP project through a private contract.
The suppliers have been determined as ▲Pfizer (3.28 million doses) ▲Moderna (2.02 million doses), with HK inno.N and Boryung Biopharma, respectively, serving as the domestic distributors (companies with exclusive sales rights) for the COVID-19 vaccines. The vaccination will target high-risk groups, including the elderly aged 65 and over and immunocompromised individuals aged 6 months and older. Vaccinations are scheduled to begin in mid-October.
This transition of the COVID-19 vaccination program to the NIP is analyzed as an effort to increase the efficiency and predictability of the vaccination system, as the demand for vaccination among high-risk groups persists.
The key to this transition is the establishment of a 'seasonal vaccination system'. Previously, vaccination plans were adjusted as needed based on the COVID-19 epidemic situation and vaccine supply conditions. However, after the NIP transition, it is expected that routine vaccinations will be standardized, like the flu vaccine.
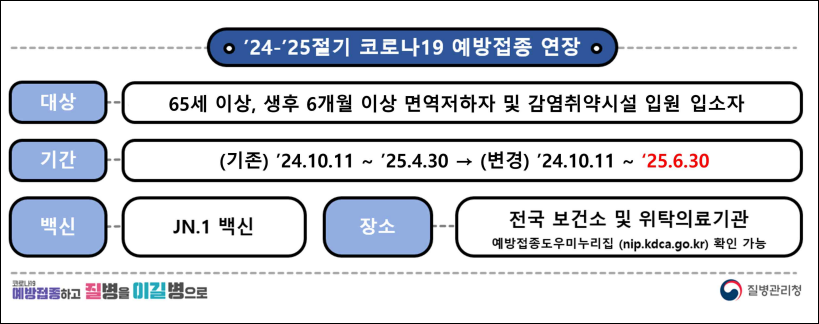
Accordingly, the vaccination implementation guidelines, recommendation criteria, and vaccine distribution are also expected to be managed solely within the government system. For the 2024-2025 seasonal COVID-19 vaccine, vaccinations were administered from October 11th to April 30th.
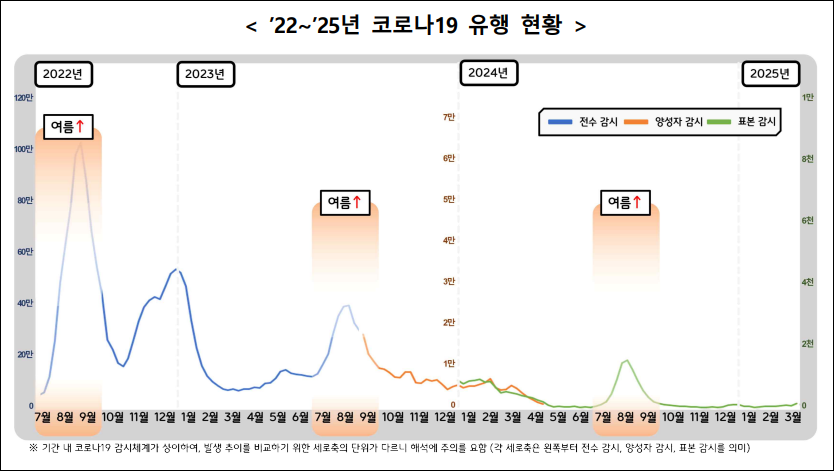
However, as the incidence of COVID-19 increased not only in winter but also in summer, the vaccination period was extended from late April to June 30 of this year.
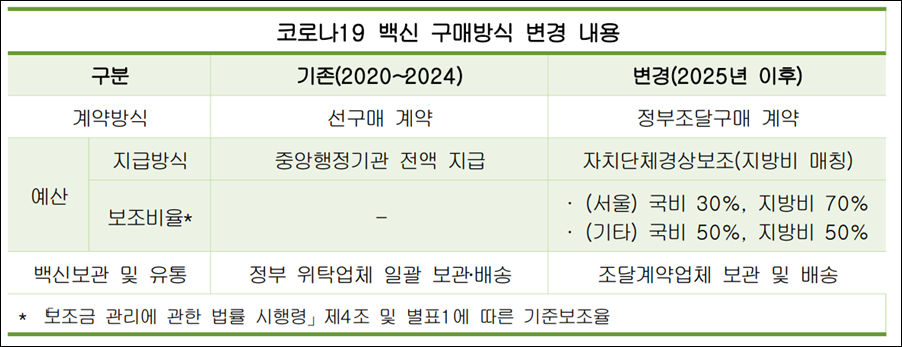
The financial structure will also change. Unlike the previous system of full state funding, a 'national+local government matching fund' model will be introduced for this NIP project.
The funding will consist of 30% national and 70% local government funds for Seoul, and 50% national and 50% local government funds for other regions. Since each local government will be responsible for executing the budget and managing vaccination rates, there is a possibility of differences in campaign intensity and implementation between regions.
Less Uncertainty for Pharmaceutical Suppliers...Expectations for Future Expansion of Vaccination Targets
For vaccine suppliers Pfizer and Moderna, the latest structural change is expected to be a favorable shift in terms of supply stability and business predictability.
Previously, COVID-19 vaccinations were based on government pre-purchase, with storage and delivery handled by government-commissioned companies. Such a system had possibility that the project could be terminated at any time based on the government's will.
Now that it is included in the NIP, a stable volume of vaccines will be secured, and production and supply plans can be formulated based on an annual procurement cycle.
From the government's perspective, even though the procurement is based on a private contract, price competitiveness and supply capabilities are included as evaluation factors, ensuring transparency in the supplier selection process.
However, the responsibilities of pharmaceutical companies are also expected to increase. Since manufacturers will be directly responsible for storage and delivery, the company's logistical capabilities, including maintaining the cold chain, handling returns, and managing product exchanges, will be a critical variable.
Although NIP vaccines have relatively fewer marketing elements, the availability of two vaccine options, along with the allowance for exchanges of vaccines nearing their expiration date and returns of up to 5%, means there is a high possibility of future competition between pharmaceutical companies.
In the clinical field, there is an expectation for a unified system, along with a focus on whether the target population will be expanded in the future. Currently, the program targets only high-risk groups, but it could be extended to the general population, similar to the flu (influenza), as the vaccination program becomes more established.
First, experts advise that a practical strategy is needed to improve vaccination rates alongside the implementation of the NIP.
Professor Woo Joo Kim of Korea University Guro Hospital's Department of Infectious Diseases stated in a meeting with DailyPharm, "High-risk groups, such as the elderly and those with underlying diseases, must be aware of their risk of infection and proactively get vaccinated. If they have symptoms, they must seek diagnosis and treatment quickly." Kim added, "At a societal level, a 'culture of resting when sick' needs to be established, and at a government level, an independent surveillance system for COVID-19 alone needs to be built."
Kim also added, "Data on the latest COVID-19 epidemic trends, disease burden, and vaccine efficacy and safety must be understood to formulate vaccination timing and public relations strategies swiftly."
-

- 0
댓글 운영방식은
댓글은 실명게재와 익명게재 방식이 있으며, 실명은 이름과 아이디가 노출됩니다. 익명은 필명으로 등록 가능하며, 대댓글은 익명으로 등록 가능합니다.
댓글 노출방식은
댓글 명예자문위원(팜-코니언-필기모양 아이콘)으로 위촉된 데일리팜 회원의 댓글은 ‘게시판형 보기’와 ’펼쳐보기형’ 리스트에서 항상 최상단에 노출됩니다. 새로운 댓글을 올리는 일반회원은 ‘게시판형’과 ‘펼쳐보기형’ 모두 팜코니언 회원이 쓴 댓글의 하단에 실시간 노출됩니다.
댓글의 삭제 기준은
다음의 경우 사전 통보없이 삭제하고 아이디 이용정지 또는 영구 가입제한이 될 수도 있습니다.
-
저작권·인격권 등 타인의 권리를 침해하는 경우
상용 프로그램의 등록과 게재, 배포를 안내하는 게시물
타인 또는 제3자의 저작권 및 기타 권리를 침해한 내용을 담은 게시물
-
근거 없는 비방·명예를 훼손하는 게시물
특정 이용자 및 개인에 대한 인신 공격적인 내용의 글 및 직접적인 욕설이 사용된 경우
특정 지역 및 종교간의 감정대립을 조장하는 내용
사실 확인이 안된 소문을 유포 시키는 경우
욕설과 비어, 속어를 담은 내용
정당법 및 공직선거법, 관계 법령에 저촉되는 경우(선관위 요청 시 즉시 삭제)
특정 지역이나 단체를 비하하는 경우
특정인의 명예를 훼손하여 해당인이 삭제를 요청하는 경우
특정인의 개인정보(주민등록번호, 전화, 상세주소 등)를 무단으로 게시하는 경우
타인의 ID 혹은 닉네임을 도용하는 경우
-
게시판 특성상 제한되는 내용
서비스 주제와 맞지 않는 내용의 글을 게재한 경우
동일 내용의 연속 게재 및 여러 기사에 중복 게재한 경우
부분적으로 변경하여 반복 게재하는 경우도 포함
제목과 관련 없는 내용의 게시물, 제목과 본문이 무관한 경우
돈벌기 및 직·간접 상업적 목적의 내용이 포함된 게시물
게시물 읽기 유도 등을 위해 내용과 무관한 제목을 사용한 경우
-
수사기관 등의 공식적인 요청이 있는 경우
-
기타사항
각 서비스의 필요성에 따라 미리 공지한 경우
기타 법률에 저촉되는 정보 게재를 목적으로 할 경우
기타 원만한 운영을 위해 운영자가 필요하다고 판단되는 내용
-
사실 관계 확인 후 삭제
저작권자로부터 허락받지 않은 내용을 무단 게재, 복제, 배포하는 경우
타인의 초상권을 침해하거나 개인정보를 유출하는 경우
당사에 제공한 이용자의 정보가 허위인 경우 (타인의 ID, 비밀번호 도용 등)
※이상의 내용중 일부 사항에 적용될 경우 이용약관 및 관련 법률에 의해 제재를 받으실 수도 있으며, 민·형사상 처벌을 받을 수도 있습니다.
※위에 명시되지 않은 내용이더라도 불법적인 내용으로 판단되거나 데일리팜 서비스에 바람직하지 않다고 판단되는 경우는 선 조치 이후 본 관리 기준을 수정 공시하겠습니다.
※기타 문의 사항은 데일리팜 운영자에게 연락주십시오. 메일 주소는 dailypharm@dailypharm.com입니다.
- [Op-Ed] Patients, no time left for 'new drug comb therapies'
- Special Contribution | Eo, Yun-Ho









