- Policy
- MFDS says, abortion drug approval cannot be reviewed
- by Lee, Hye-Kyung Jul 30, 2025 06:16am
- Hyundai Pharm filed a marketing authorization application for oral abortion drug 'Mifegymiso Tab (mifepristone·misoprostol)' last year. However, it has been confirmed that, under the current state of legislative void, the review cannot even begin. On the 30th, the Ministry of Food and Drug Safety's (MFDS) Pharmaceutical Approval Manageme
- Company
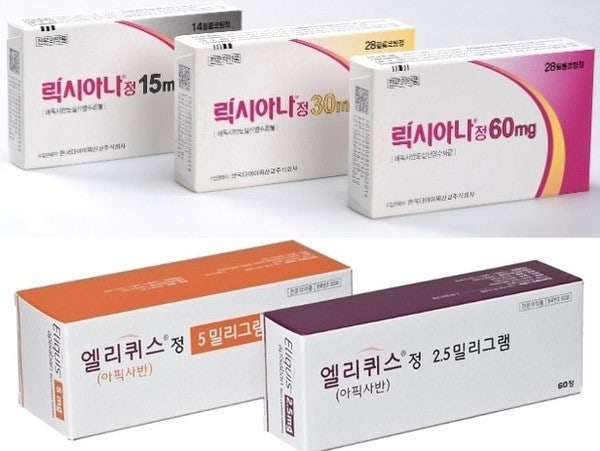
- Lixiana solely leads DOAC mkt… Xarelto, Eliquis↓
- by Kim, Jin-Gu Jul 30, 2025 06:16am
- Original direct-acting oral anticoagulant (DOAC) drugs have faced mixed fortunes in the Korean market. Prescription sales of Lixiana (edoxaban) increased by 8% year-on-year, solidifying its lead in Korea’s market. Its market share in the DOAC market expanded to 49%, and the analysis is that Lixiana will continue its lead in the market
- Product
- Platforms promote Wegovy's lowest price is ₩390,000
- by Kang, Hye-Kyung Jul 30, 2025 06:10am
- Non-face-to-face treatment platforms are facing backlash from pharmacies after launching activities promoting the lowest prices for non-reimbursable drugs. Doctornow, a leading non-face-to-face medical consultation platform, is being criticized for encouraging price competition for non-reimbursable drugs and causing confusion among consumers.
- Company
- Expanded reimb for Jardiance…management of CRM disease
- by Whang, byung-woo Jul 30, 2025 06:10am
- Boehringer Ingelheim announced on the 29th that its SGLT2 inhibitor, Jardiance (empagliflozin), will be reimbursed by the National Health Insurance for the treatment of chronic kidney disease (CKD), starting August 1, according to the Ministry of Health and Welfare notification. According to this notification, Jardiance will be reimbursed
- Company
- Neurophet partners with Roche for AI tech verification
- by Whang, byung-woo Jul 30, 2025 06:10am
- Neurophet (Co-CEO Jun-gil Bin, Dong-hyun Kim), a specialized AI company in brain disease diagnosis and treatment, announced on the 29th that it has officially signed a joint research agreement with Roche and has begun full-scale collaboration Neurophet has already been sharing data with Roche, and with the official announcement of the col
- Company
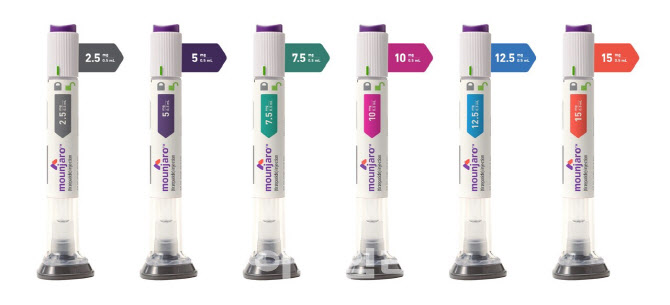
- Attention drawn to reimb status of 'Mounjaro' for diabetes
- by Moon, sung-ho Jul 30, 2025 06:09am
- As the official launch of Mounjaro (tirzepatide, Eli Lilly Korea), approved in Korea as an adjunct therapy for chronic weight management following its indication for adult type 2 diabetes, is imminent, the reimbursement status of the drug is garnering attention. The focus is on whether it will be recognized and reimbursed as the first 'innova
- Policy
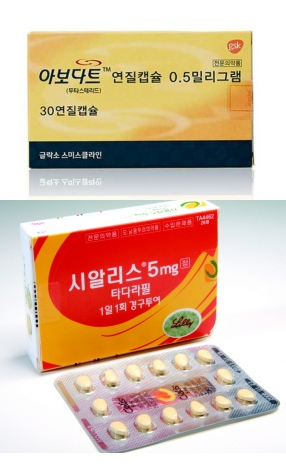
- Will the Korean Avodart+Cialis combos be reimbursed?
- by Lee, Tak-Sun Jul 29, 2025 06:04am
- There is growing interest in whether domestic pharmaceutical companies will succeed in securing reimbursement for the world's first dutasteride and tadalafil combination drug. Currently, this drug is undergoing a new drug reimbursement evaluation process rather than the standard combination drug assessment, as tadalafil is not a reimbursed
- Company
- "A paradigm shift in ulcerative colitis treatment"
- by Whang, byung-woo Jul 29, 2025 06:04am
- The treatment strategy for ulcerative colitis is rapidly evolving with the emergence of new global drugs. Global guidelines recommend a rapid transition to advanced therapies (ATs) early on if 5-ASA treatment fails. In line with these recommendations, Korea also needs a shift in its treatment paradigm. However, the early utilization of hig
- Company
- Fewer drugs reimbursed 5 years into pricing reform
- by Chon, Seung-Hyun Jul 29, 2025 06:04am
- Over the past five years, 4,500 drugs have been removed from the reimbursement list in Korea. The number of new drugs entering the market has decreased significantly with the introduction of generic drug price reforms and joint development regulations. The number of prescription drug approvals has decreased by more than 80% compared to 5 years a
- Company
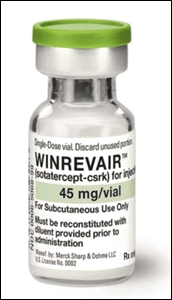
- 1st drug to win 2nd concurrent approval·pricing program
- by Eo, Yun-Ho Jul 28, 2025 06:08am
- As the first drug has been approved under the 2nd concurrent approval·drug pricing program, the process for insurance reimbursement is garnering attention. MSD Korea's 'Winrevair (sotatercept),' a new drug for pulmonary arterial hypertension (PAH), recently obtained final approval from the Ministry of Food and Drug Safety. As it has be