- Company
- "Aiming the Alzheimer's market with ultrasound"
- by Whang, byung-woo May 22, 2025 06:07am
- Korea's biotech company Deepsonbio is gaining interest for its ultrasound-based brain disorder treatment technology. The company's recent exploratory clinical trials demonstrated the effect of improving cognitive function for patients with Alzheimer's disease and those with normal pressure hydrocephalus. Deepsonbio plans to enter the market
- Company
- Dyslipidemia drug 'Leqvio' enters drug price negotiatiation
- by Eo, Yun-Ho May 21, 2025 06:37am
- Leqvio, a new drug for dyslipidemia administered once yearly, has recently entered into negotiations with the National Health Insurance Service (NHIS). In February, Novartis Korea's siRNA drug Leqvio (inclisiran) had initially failed at setting criteria for 'reducing cardiovascular events in patients with atherosclerotic cardiovascular d
- Company
- ‘Bispecific antibodies gain attention as option for DLBCL'
- by Whang, byung-woo May 21, 2025 06:36am
- Diffuse large B-cell lymphoma (DLBCL) is considered a difficult disease to treat due to its aggressive nature, but the emergence of new drugs has brought about a paradigm shift in its treatment. As efforts for DLBCL progress towards a cure beyond extending survival, the role of bispecific antibody therapies is garnering attention. Georg Le
- Policy
- 'AI-enabled stem cell for pediatric epilepsy proves effect'
- by Lee, Hye-Kyung May 21, 2025 06:36am
- A new treatment possibility has opened up for pediatric epilepsy patients who have shown little response to existing treatments. The Korea Health Industry Development Institute (President: Soondo Cha) announced on the 19th that Professor Hoon-Chul Kang’s research team at Yonsei University Severance Children's Hospital has successfully disco
- Company
- Trodelvy may be the 1st drug reimbursed with ICER benefits
- by Eo, Yun-Ho May 21, 2025 06:36am
- ADC breast cancer drug Trodelvy may soon be listed for insurance reimbursement in Korea. Gilead Sciences recently finalized price negotiations with the National Health Insurance Service for its triple-negative breast cancer (TNBC) treatment Trodelvy (sacituzumab govitecan). As a result, Trodelvy is scheduled to be presented at the Heal
- Company
- MSD expands domestic clinical trial cooperation
- by Whang, byung-woo May 20, 2025 06:00am
- MSD Korea has broken its record for the most clinical trial approvals in Korea and is now in full swing, developing innovative new drugs for Koreans. With open innovation playing an increasingly important role in new drug research and development (R&D), MSD is expanding its ties with Korea, which plays a pivotal role in its global clinical tr
- Policy
- Citus generics price raised, Ameliebou Inj huge price cut
- by Lee, Tak-Sun May 20, 2025 06:00am
- Prices of Citus generic drugs will be raised. As the price of original Citus has been adjusted, the prices of generic drugs that were reimbursement-listed in January have been recalculated. Meanwhile, the price of Samsung Bioepis' Lucentis biosimilar 'Ameliebou Inj' has been substantially cut, resulting in a significant difference from the or
- Policy
- MFDS to review reference drug application every 2 months
- by Lee, Hye-Kyung May 20, 2025 05:59am
- The change in the procedure for selecting reference drugs for pharmaceutical equivalence tests is being well received by the domestic pharmaceutical industry. The Ministry of Food and Drug Safety recently announced a revision to the 'Guideline for Selecting Reference drugs for Pharmaceutical Equivalence Tests' and is seeking opinions on the c
- Company
- Hanmi-MSD collaborate for R&D
- by Cha, Jihyun May 20, 2025 05:59am
- Hanmi Pharmaceutical entered into a clinical trial collaboration agreement with the U.S.-based Merck (MSD) for developing an immune anticancer drug candidate. The clinical collaboration between Hanmi Pharmaceutical (hereafter, Hanmi) and Merck has expanded to three cases. In addition to clinical trial collaboration, Hanmi continues to collaborat
- Company
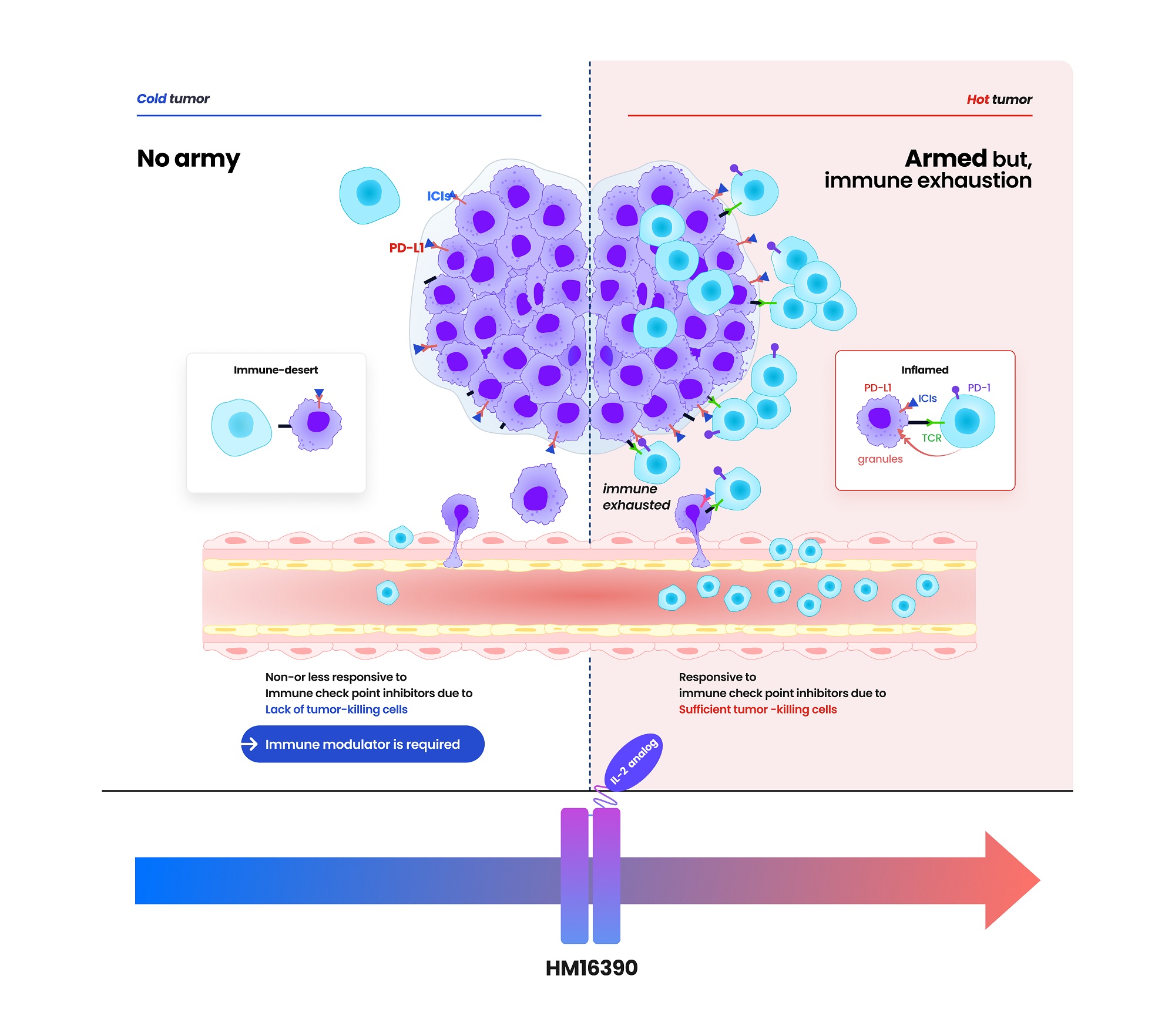
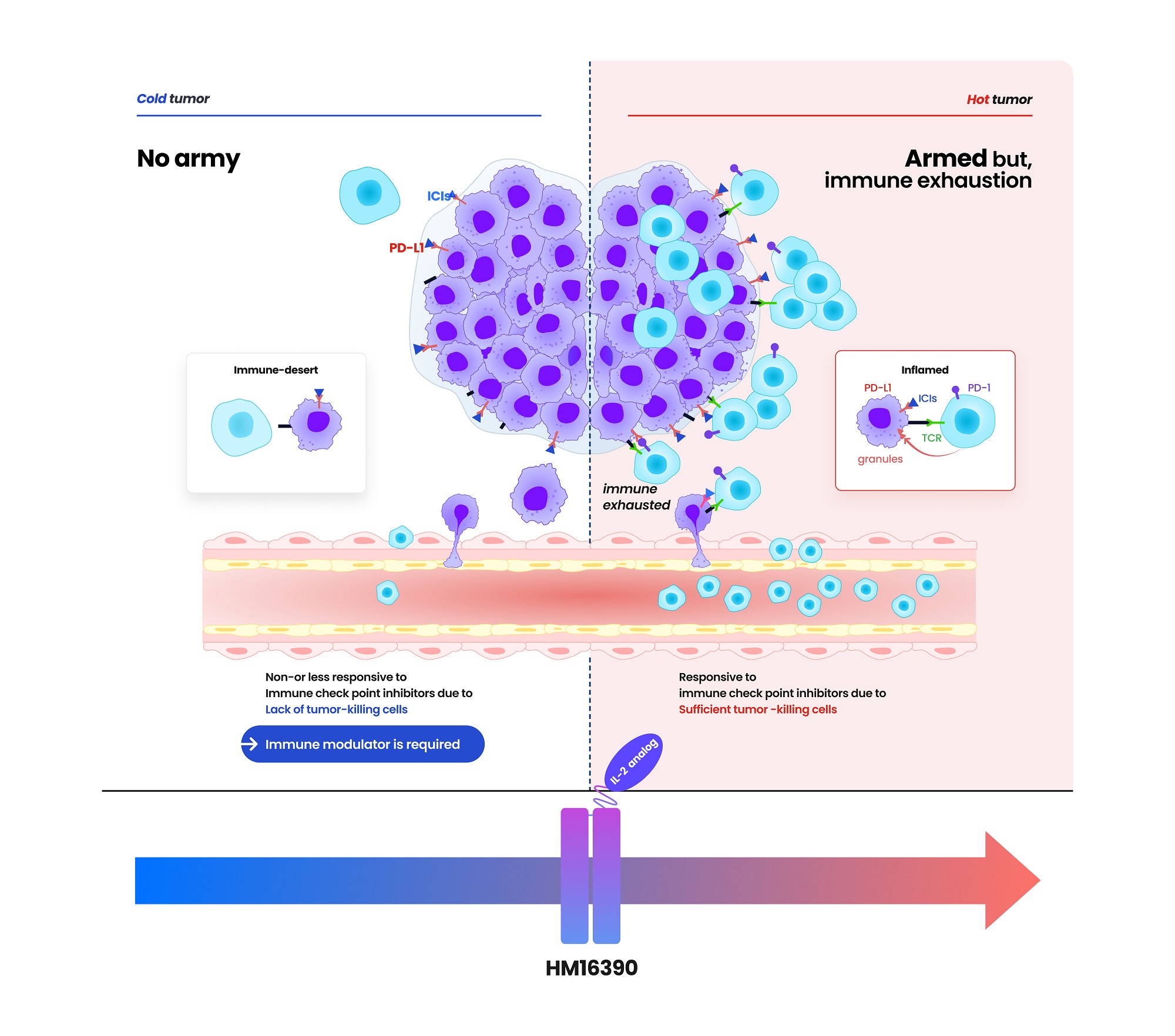
- Hanmi partners with MSD for next-gen IL-2 analog development
- by Cha, Jihyun May 20, 2025 05:58am
- Hanmi Pharmaceutical (CEO: Jae-Hyun Park) announced on the 19th that it has signed a clinical trial collaboration and supply agreement with U.S. Merck (MSD) to evaluate the combination therapy of its LAPS IL-2 analog 'HM16390' and MSD's anti-PD-1 immunotherapy 'Keytruda' (pembrolizumab). Hanmi Pharmaceutical will sponsor and oversee the Phase