- Policy
- MOHW “Not to worry vaccine shortage, delay and safety”
- by Lee, Jeong-Hwan Dec 15, 2020 06:03am
- The South Korea’s Ministry of Health and Welfare (MOHW) official says the concerns about the shortage or delay in COVID-19 vaccine distribution or AstraZeneca vaccine’s safety issue would be unnecessary. The official claimed the government has already secured vaccine doses for total 44 million people through COVAX Facility and privat
- Company
- Evenity, customized for patients with high risk of fracture
- by Dec 15, 2020 06:03am
- As Amgen's new osteoporosis treatment, Evenity (Romosozumab) was applied from this month, it could be used more by patients. In particular, Evenity, which has proven its strong efficacy in patients with high risk of fracture, may be useful. On the 10th, Amgen held an online meeting to commemorate the launch of Evenity insurance benefits, a
- Company
- The market for erectile dysfunction drugs is on the rise
- by Chon, Seung-Hyun Dec 15, 2020 06:03am
- The market for erectile dysfunction treatments is recovering. The market size contracted in the aftermath of COVID-19 in the first half, but it rebounded in the third quarter. Generic for Cialis have been largely succeeful. According to IQVIA, a drug research institute on the 10th, the market for erectile dysfunction treatments in the third
- Opinion
- [Reporter’s View] Notes on seeking for COVID-19 vaccine
- by An, Kyung-Jin Dec 14, 2020 05:59am
- The novel coronavirus disease-19 (COVID-19) is spreading at a concerning rate in South Korea. Although the social distancing level was raised to 2.5 two weeks after the government ordered the social distancing level 2 on Nov. 24, the third wave of pandemic seems to keep rising. The authority is even considering on raising the level up to 3,
- Company
- Pfizer's Xeljanz XR (once a day), approved in Korea
- by Dec 14, 2020 05:58am
- Pfizer Korea (CEO Dong-wook Oh) &8203;&8203;announced on the 10th that it has received approval for Xeljanz XR 11 mg, a rheumatoid arthritis treatment, from the MFDS on the 7th. Xeljanz XR is a sustained-release tablet formulation of the existing Xeljanz 5mg (Tofacitinib). It can be used for the treatment of moderate to severe active rhe
- Policy
- The vaccination fee for COVID-19 will be free
- by Kang, Shin-Kook Dec 14, 2020 05:57am
- The vaccination fee is free in principle, and the elderly and medical staff will be given priority. IT starts from February to March next year as early as possible. The inoculation rate of 60-70% for the nation to become immune is expected to reach the second half of next year. Young-Rae Son, Central Disaster Management Headquarters' plann
- Company
- Bavencio, difficulty in expanding indication
- by Dec 11, 2020 06:16am
- The possibility of approval for a renal cell carcinoma indication for Bavencio(Avelumab), an immunotherapy developed by Merck and Pfizer, is very low. This is because the Central Pharmaceutical Affairs Review Committee of the MFDS has not recognized Bavencio's therapeutic effect. The Central Pharmaceutical Affairs Review Committee held a m
- Opinion
- [Reporter’s View] Real reasons for disqualification?
- by Eo, Yun-Ho Dec 11, 2020 06:15am
- A multinational pharmaceutical company Sanofi Korea has failed to qualify as a certified innovative pharmaceutical company. Fairness aside, the transparency in the process seems questionable. Since its first certification won in 2014, the company lost its certification status after six years. Now, only three multinational companies&8212
- Product
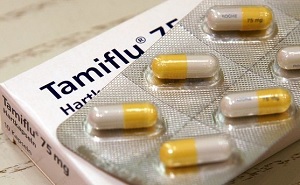
- Instructing Tamiflu users on neurological side effect
- by Kang, Shin-Kook Dec 11, 2020 06:14am
- “Have you heard of that incident two years ago, where a middle school student took a fall after taking Tamiflu?” The importance of influenza treatment administration instruction is reiterated due to the reported neurological adverse reaction after the administration. On Dec. 9, the Korean Pharmaceutical Association (KPA) notified a
- Policy
- HIRA green lit coverage on 14 out of 26 new drugs
- by Lee, Hye-Kyung Dec 10, 2020 06:11am
- 14 out of 26 new drugs deliberated by the Health Insurance Review and Assessment Service (HIRA) Drug Reimbursement Evaluation Committee (DREC) were listed for the healthcare reimbursement. To this date, the DREC has listing rate of 53.8%, but it could get higher as there are new drugs, cleared by DREC during the 10th meeting convened i