- Policy
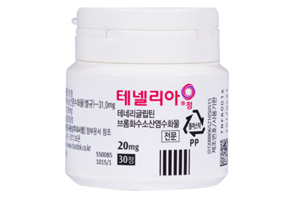
- Handok expands its Tenelia combo lineup
- by Lee, Hye-Kyung Mar 7, 2025 05:56am
- Handok is speeding up the development of a combination drug that combines DPP-4 inhibitor diabetes treatment Tenelia (teneligliptin hydrobromide hydrate)' with SGLT-2 inhibitor 'Jadiance (Empagliflozin). On the 4th, the Ministry of Food and Drug Safety approved an open-label, randomized, empty stomach, single orally administered, two-arm,
- Opinion
- [Reporter's View] Concerns about new drugs for rare diseases
- by Son, Hyung Min Mar 7, 2025 05:56am
- Duchenne muscular dystrophy is a rare muscular disorder caused by the lack of dystrophin, primarily affecting boys. The symptoms typically begin before the age of 3 and rapidly deteriorate, eventually leading to the loss of ability to walk before the age of 10 and above. To date, various genetic targeted therapies for Duchenne muscular d
- Company
- Roche adds expertise through leadership appointments
- by Whang, byung-woo Mar 7, 2025 05:56am
- Roche Korea is strengthening its leadership by appointing new leads to its Oncology and Hematology cluster and Specialty Medicines cluster. The company announced on the 5th that it has appointed Jinyoung Jeong as the new head of the Oncology·Hematology Cluster and Hyun-mi Kim as the new head of the Specialty Medicines Cluster. By appo
- New drug 'Wainua' for amyloid polyneuropathy receives ODD
- by Eo, Yun-Ho Mar 7, 2025 05:55am
- A new drug, 'Wainua,' for the treatment of amyloid polyneuropathy has been designated as an orphan drug in South Korea. On March 5, the Ministry of Food and Drug Safety (MFDS) notified of the orphan drug The drug is indicated for the treatment of hereditary transthyretin-mediated amyloidosis (ATTRv-PN). Wainua (eplontersen) is a t
- Company
- SK Chemical signs a joint sales agreement with Viatris
- by Lee, Seok-Jun Mar 6, 2025 05:58am
- SK Chemicals, which developed the first natural drug for osteoarthritis in Korea, ‘Joins Tab,’ has secured additional pain medications for its portfolio. SK Chemical (CEO Jae-Hyun Ahn) announced on the 5th that it has signed a distribution and sales agreement with Viatris Korea for △Lyrica, △ Neurontin, and △ Celebrex. Under the
- Company
- Vyndamax is reimbursed for ATTR-CM in Korea
- by Whang, byung-woo Mar 6, 2025 05:58am
- Pfizer Korea announced on the 5th that its Vyndamax (tafamidis), a treatment for wild-type or hereditary transthyretin amyloidosis cardiomyopathy (ATTR-CM), has been granted reimbursement by Korea’s National Health Insurance. ATTR-CM is a progressive rare disease in which the naturally circulating transport protein in the blood, transthy
- Company
- Novo Nordisk Korea appoints Kasper Roseeuw Poulsen as new GM
- by Whang, byung-woo Mar 6, 2025 05:58am
- On March 5, Novo Nordisk Korea announced the appointment of Kasper Roseeuw Poulsen as the new General Manager starting March 2025. The new GM Kasper Roseeuw Poulsen joined Novo Nordisk in 2006 and served key roles in finance, strategy, organizational development, commercial partnership, and management across Europe, South America, and Asi
- Company
- Pfizer launches JAK inhibitor for severe alopecia areata
- by Whang, byung-woo Mar 6, 2025 05:58am
- Pfizer Korea will launch a new Janus kinase (JAK) inhibitor treatment Litfulo (ritlecitinib tosilate), and set to challenge the market for severe alopecia areata. Previously launched Olumiant (baricitinib), the first drug approved in South Korea for treating adult patients with severe alopecia areata, has already settled in the market. The co
- Policy
- CKD and Daewoong Bio enter donepezil-memantine combo market
- by Lee, Tak-Sun Mar 6, 2025 05:55am
- Chong Kun Dang Pharmaceutical and Daewoong Bio will enter the donepezil-memantine combination drug market that was launched this month. Although Chong Kun Dang and Daewoong Bio’s products were not included in the initial list of approved products, the companies will join the market in the form of a license transfer and co-promotion agre
- Company
- "COVID-19 response has changed to protect high risk groups"
- by Whang, byung-woo Mar 5, 2025 06:01am
- As COVID-19 transitioned to the endemic phase, an important issue has arisen regarding 'how' to prevent and respond to the disease, unlike the initial period focused only on response. One of the big changes was the reimbursement of treatments. On October 25, 2024, The Ministry of Health and Welfare (MOHW) approved the National Health Insuranc