- Policy
- Hanmi’s BTK inhibitor poseltinib approved for P1T in KOR
- by Lee, Hye-Kyung Jul 11, 2025 06:09am
- Hanmi Pharmaceutical's BTK inhibitor ‘poseltinib,’ which was licensed out to Nobo Medicine last year, will enter Phase I clinical trials in Korea as a monotherapy. On the 7th, the Ministry of Food and Drug Safety approved an open, multicenter, monotherapy, dose escalation Phase I clinical trial of NB02 (poseltinib) for patients with rel
- Policy
- Discussion on "Indication-specific drug pricing system"
- by Lee, Tak-Sun Jul 10, 2025 06:09am
- The Health Insurance Review & Assessment Service (HIRA) announced that the issue of 'indication-specific drug pricing system' proposed by the pharmaceutical industry may require further discussion, and the immeditate implementation is difficult. They explained that further discussion is needed, particularly regarding the prevention of co
- Policy
- Introduction of AI for drug approval and review in Korea
- by Lee, Hye-Kyung Jul 10, 2025 06:09am
- The Ministry of Food and Drug Safety is conducting follow-up research to introduce generative artificial intelligence (AI) to the domestic drug approval and review field starting next year. The Pharmaceutical and Medical Device Research Department of the National Institute of Food and Drug Safety Evaluation is conducting an ISP project to est
- Policy
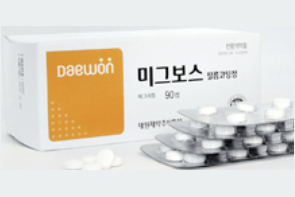
- Daewoong’s generic version of Migbose approved in Korea
- by Lee, Hye-Kyung Jul 9, 2025 06:09am
- Daewoong Pharmaceutical has been approved to sell a generic version of the diabetes treatment Migbose Film-Coated Tablets (miglitol) in Korea. On the 7th, the Ministry of Food and Drug Safety approved Daewoong Pharmaceutical's Daewoong Miglitol Tab. (miglitol). Like the original Migbose, Daewoong Miglitol Tab is a film-coated tablet indica
- Policy
- Industry requests postponement of drug reimbursement reevals
- by Lee, Tak-Sun Jul 8, 2025 06:37am
- The pharmaceutical industry has expressed the need to postpone next year's drug reimbursement reevaluations to the government. The reason is that the reevaluation targets have not been decided by the second half of the year, leaving insufficient time for the target companies to prepare the necessary data. As a result, some are even sugges
- Policy
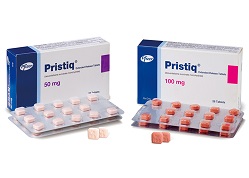
- Low-strength salt-modified Pristiq now available
- by Lee, Tak-Sun Jul 8, 2025 06:35am
- Companies with salt-modified formulations of the antidepressant Pristiq (desvenlafaxine succinate monohydrate) are strengthening their market competitiveness with a 25mg low-strength product that had not been available in the original or generic formulations. With price adjustments near due to the entry of generics, attention is focused o
- Policy
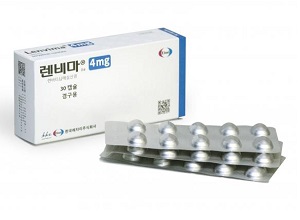
- Boryung's follow-on Lenvima, 'Lenvanib,' becomes reimbursed
- by Lee, Tak-Sun Jul 7, 2025 06:09am
- Boryung has successfully obtained reimbursement listing for all dosages of 'Lenvanib Cap (lenvatinib mesylate dimethyl sulfoxide),' its follow-on drug to the anti-cancer medication Lenvima (lenvatinib mesylate, Eisai), which is used to treat conditions such as liver cancer. With the patent dispute with the original manufacturer, Eisai, sti
- Policy
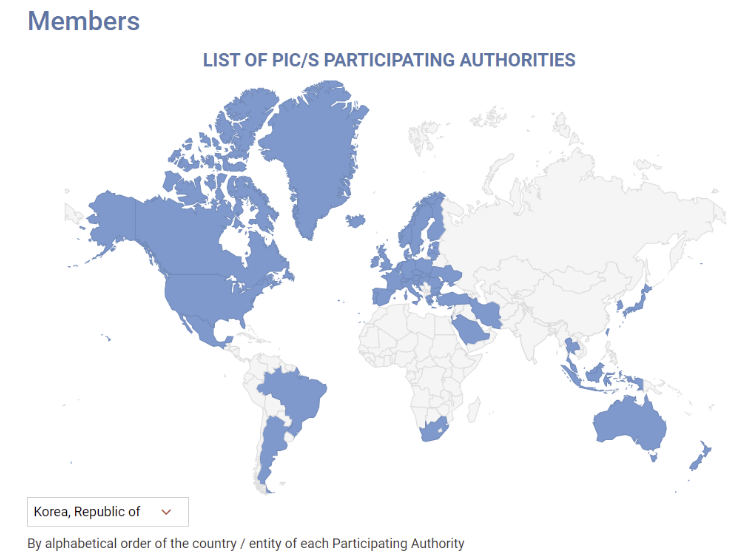
- MFDS reviews GMP standards for sterile preparations abroad
- by Lee, Hye-Kyung Jul 7, 2025 06:09am
- With the Ministry of Food and Drug Safety planning to enforce revised GMP standards for sterile drug products reflecting PIC/S international standards in December, it plans to identify research tasks on items that require international harmonization by analyzing the regulatory status of reference countries. Ahead of its re-entry into PIC/S in
- Policy
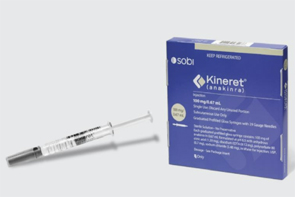
- MFDS reviews emergency import of 'anakinra'
- by Lee, Hye-Kyung Jul 7, 2025 06:08am
- The Ministry of Food and Drug Safety (MFDS) is reportedly conducting a review of whether to urgently import anakinra (brand name Kineret) for the treatment of severe adverse reactions associated with CAR-T cell therapy. On June 2nd, in response to an official inquiry from specialized journalists, MFDS stated, "MFDS is currently reviewing
- Policy
- National Office of Investigation announces rebate crackdown
- by Lee, Jeong-Hwan Jul 4, 2025 06:06am
- Amidst the National Police Agency's announcement of a special crackdown on illegal drug rebates following the launch of the new administration, the Ministry of Health and Welfare is also busy assessing the situation. The MOHW plans to actively consult with the National Police Agency if it receives a specific request for cooperation to crac