- Company
- Reimb for Perjeta as adjuvant therapy under CDDC review
- by Eo, Yun-Ho Oct 29, 2025 06:12am
- The expansion of reimbursement coverage for Perjeta’s use as adjuvant therapy in breast cancer is drawing attention. According to industry sources, Roche Korea’s HER2-positive breast cancer treatment Perjeta (pertuzumab) has been submitted for review by the Health Insurance Review and Assessment Service (HIRA) Cancer Disease Deliberatio
- Company
- Will the Indian API cause problems?
- by Kim, Jin-Gu Oct 29, 2025 06:12am
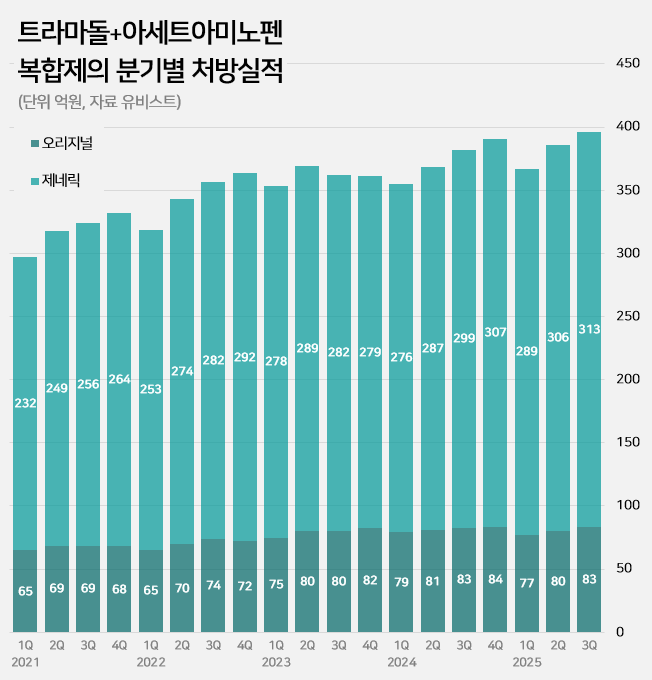
- An impurity issue has emerged as a major variable in the tramadol-acetaminophen combination drug market, valued at approximately KRW 150 billion annually. Following the detection of impurities in three generic products using Indian-sourced active pharmaceutical ingredients (API), the pharmaceutical industry is watching closely for the potenti
- Company
- Fruzaqla expands its role to late-stage colorectal cancer
- by Son, Hyung Min Oct 28, 2025 06:12am
- A new treatment option has been added to the field of metastatic colorectal cancer, which is classified as a refractory condition. Takeda Korea held a press conference on the 27th at the Plaza Hotel in Jung-gu, Seoul, to commemorate the expanded indication of its colorectal cancer drug, Fruzaqla (fruquintinib). Approved in March as a fourt
- Company
- Hanmi "Phase 3 trial confirms efficacy of GLP-1 drug"
- by Cha, Jihyun Oct 28, 2025 06:11am
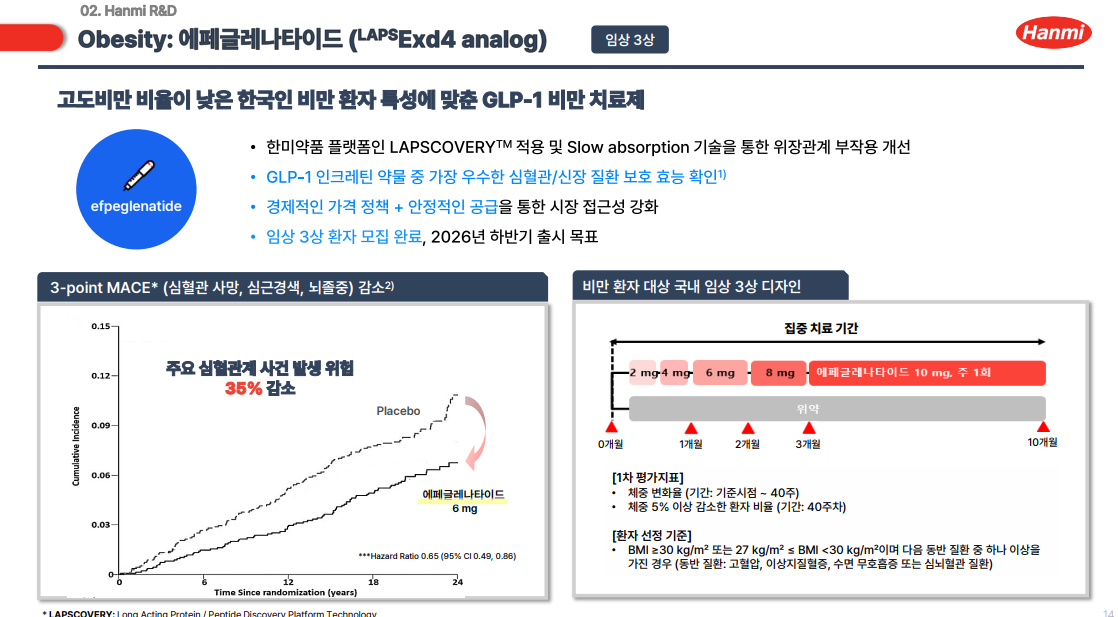
- Hanmi Pharmaceutical announced on October 27 that its glucagon-like peptide-1 (GLP-1) obesity treatment candidate, 'HM11260C' (efpeglenatide), met primary endpoints in a Phase 3 top-line study in Korea. The results confirmed significant weight-loss efficacy and safety compared with placebo. This Phase 3 trial involved 448 non-diabetic ad
- Company
- ADC offers new hope in TNBC treatment gap
- by Hwang, byoung woo Oct 28, 2025 06:11am
- The TROPION-Breast02 study presented at the European Society for Medical Oncology Congress 2025 (ESMO 2025) marks a new turning point in the treatment of triple-negative breast cancer (TNBC). As the first antibody-drug conjugate (ADC) trial to significantly improve both overall survival (OS) and progression-free survival (PFS) in patients who
- Company
- Ultomiris's reimb standards eased for aHUS
- by Son, Hyung Min Oct 27, 2025 06:11am
- The reimbursement criteria for C5 complement inhibitors in atypical hemolytic uremic syndrome (aHUS) have been eased this month. With this improvement, as the previous criteria had stringent reimbursement conditions, such as the requirement for prior review, patient access is expected to improve. On the 24th, AstraZeneca Korea held a brie
- Company
- ImmuneOncia speeds commercialization with product-fit plan
- by Hwang, byoung woo Oct 27, 2025 06:10am
- ImmuneOncia is concretizing its ‘commercialization strategy that starts with rare cancers’ as entry points for its immuno-oncology portfolio. ImmuneOncia, a subsidiary of Yuhan Corp that specializes in immuno-oncology, presented new clinical results on its PD-L1 antibody IMC-001 (danvastotug) and CD47 antibody IMC-002 at the European Societ
- Company
- AstraZeneca set for leadership shift after 2 years
- by Eo, Yun-Ho Oct 27, 2025 06:10am
- AstraZeneca Korea sees a leadership shift after approximately two years. According to industry sources, Sehwan Chon (51), CEO of AstraZeneca Korea, will resign at the end of this month (October). Consequently, Eldana Sauran, currently a Regional Commercial Director of Oncology Unit at AstraZeneca, is expected to be appointed as the int
- Company
- "Childhood obesity is the starting point for adult diseases"
- by Son, Hyung Min Oct 27, 2025 06:09am
- "Obesity is no longer just a matter of appearance. Especially in growing children and adolescents, obesity is the starting point for adult diseases like diabetes and hypertension, and a factor that determines lifelong health. It must be approached as a disease, not just simple weight management." Professor Kyoung-gon Kim of Department of
- Company
- ‘Reimb targeted therapies for Stage IV gastric cancer'
- by Son, Hyung Min Oct 24, 2025 06:15am
- A targeted therapy option for gastric cancer that demonstrated exceptional survival extension benefits in Asian patients is on the verge of being listed for insurance reimbursement in Korea. If reimbursed, the medical community expects the improved access to the new drug will contribute to improving the persistently low survival rates among meta