- Policy
- First batch of drugs gain reimb through AEN pilot project
- by Jung, Heung-Jun Nov 17, 2025 06:11am
- With the first drugs under the Approval-Evaluation-Negotiation pilot program all securing reimbursement, the timing for the second batch of drugs currently under review to enter reimbursment listing is also approaching. Expectations are building for the possibility of their sequential insurance coverage as early as the first half of next
- Policy
- Pricing nego for Erleada underway…last hurdle to reimb
- by Jung, Heung-Jun Nov 14, 2025 06:12am
- Janssen Korea's prostate cancer treatment Erleada (apalutamide) has entered price negotiations, the final hurdle for its reimbursement expansion in Korea. Erleada passed the Drug Reimbursement Evaluation Committee review last October and was deemed appropriate to expand reimbursement coverage to ‘high-risk non-metastatic castration-resis
- Policy
- Ultomiris gains reimbursement approval for 'NMOSD·gMG'
- by Jung, Heung-Jun Nov 14, 2025 06:11am
- AstraZeneca's Ultomiris (ravulizumab) will receive reimbursement coverage for Neuromyelitis Optica Spectrum Disorder (NMOSD) this month, and its reimbursement scope will expand to generalized myasthenia gravis. Notably, the Ministry of Health and Welfare (MOHW) has requested reimbursement coverage for myasthenia gravis. Therefore, it was
- Policy
- Pharmaceutical post-market management system to be enhanced
- by Jung, Heung-Jun Nov 14, 2025 06:11am
- The National Health Insurance Service (NHIS) plans to revise its drug price negotiation and post-market management systems next year, with a focus on stable pharmaceutical supply. The NHIS will actively enforce penalties stipulated in its regulations if pharmaceutical companies discontinue drug supply without notification in violation of agre
- Policy
- MOHW and MOJ oppose legislating mandatory INN prescribing
- by Lee, Jeong-Hwan Nov 13, 2025 06:08am
- The Ministry of Health and Welfare and the Ministry of Justice have expressed reluctance toward a bill that would only partially mandate and enforce physician international non-proprietary name (INN) prescribing for government-designated drugs with unstable supply. Despite this being President Jae-Myung Lee’s presidential election pledge
- Policy
- Yuhan–Janssen join forces to tackle lung cancer
- by Jung, Heung-Jun Nov 12, 2025 06:19am
- As Yuhan Corporation and Janssen Korea join forces to co-promote their lung cancer treatments, securing reimbursement for the combination therapy has emerged as the next major hurdle to fully realize their market synergy. Janssen Korea’s non-small-cell lung cancer drug Rybrevant (amivantamab) was granted reimbursement as monotherapy by t
- Policy
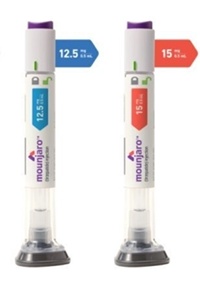
- NA requests rapid reimb listing for diabetes drug Mounjaro
- by Jung, Heung-Jun Nov 11, 2025 06:08am
- The Ministry of Health and Welfare (MOHW) announced that it will reflect the results of the October expert advisory meeting in reimbursement criteria for Mounjaro (tirzepatide), Eli Lilly’s dual GIP/GLP-1 receptor agonist, following growing calls for its rapid inclusion under the National Health Insurance for type 2 diabetes. The ministr
- Policy
- Cancer & orphan drugs apply for reimb within 1 week of apv
- by Jung, Heung-Jun Nov 10, 2025 06:10am
- Anticancer drugs and rare disease drugs that were approved in Korea this year are racing to apply for reimbursement as quickly as within a week. Six rare/serious disease treatments approved by the Ministry of Food and Drug Safety this year are undergoing reimbursement review, excluding those subject to the approval-evaluation-negotiation
- Policy
- Budget cut 20% despite increased COVID-19 vaccine uptake
- by Lee, Jeong-Hwan Nov 10, 2025 06:08am
- The Korea Disease Control and Prevention Agency (KDCA) has cut the national immunization program (NIP) budget for COVID-19 vaccines for the 2026-27 season by 20% compared to the previous year, raising societal concern over potential drops in immunity among high-risk groups like the elderly, increased mortality risk, and the possibility of ea
- Policy
- ‘No drugs are benefitting from Korea's dual pricing scheme'
- by Jung, Heung-Jun Nov 10, 2025 06:08am
- Although a separate contract system was introduced for dual pricing of drugs in March this year, following a Ministry of Health and Welfare notice, no drugs are currently subject to the dual drug pricing system, prompting the government to review expansion plans. The NHIS stated it is reviewing with the government on expanding the scope o