- Policy
- The application for selective reimb of new drugs is stalled
- by Jung, Heung-Jun Nov 7, 2025 06:17am
- Roche Korea's 'Perjeta (pertuzumab),' a breast cancer treatment, was not on the recent Cancer Disease Review Committee (CDRC) agenda, despite expectations, raising questions about the background of its exclusion. One analysis suggests that the government's policy shift toward strengthening access to rare and severe disease treatments may
- Policy
- MFDS to ease orphan drug designation eligibility criteria
- by Lee, Tak-Sun Nov 6, 2025 06:32am
- As the criteria for Orphan Drug Designation are set to be significantly eased, this is expected to expand the introduction of rare disease treatments currently not approved in Korea. The Ministry of Food and Drug Safety (MFDS) plans to revise the regulations by February of next year. The MFDS announced this during the 'Top 50 Food and
- Policy
- 'Zongertinib' for HER2-mutant NSCLC receives ODD
- by Lee, Tak-Sun Nov 5, 2025 06:25am
- Boehringer Ingelheim's 'zongertinib,' the first oral targeted therapy for patients with HER2-mutant non-small cell lung cancer (NSCLC) and which is gaining attention, received Orphan Drug Designation (ODD) from the Ministry of Food and Drug Safety (MFDS). The designation of the drug as an orphan drug is expected to accelerate its official
- Policy
- 'Rebate points deduction system' has been stalled
- by Lee, Jeong-Hwan Nov 4, 2025 06:13am
- The revision of South Korea's Innovative Pharmaceutical Company Certification System, which the Ministry of Health and Welfare (MOHW) is pursuing to foster the Korean pharmaceutical industry, is stalling. This is likely due to arguments for and against 'conversion to a rebate scoring system.' Following agreement with the pharmaceutical
- Policy
- AI-driven drug R&D key to narrowing gap with global leaders
- by Lee, Jeong-Hwan Nov 4, 2025 06:09am
- The President of the Korea Health Industry Development Institute (KHIDI stressed that Korea must strategically nurture AI and data capabilities to dramatically improve the productivity of AI-based drug R&D and rapidly close the technology gap with advanced pharmaceutical nations. He also revealed a plan to create a KRW 1-trillion mega-fun
- Policy
- Minister Jeong backs INN prescribing for shortage drugs
- by Lee, Jeong-Hwan Nov 3, 2025 06:08am
- Minister of Health and Welfare Eun-kyeong Jeong expressed support for introducing international nonproprietary name (INN) prescribing and expanding substitution dispensing by pharmacists for medicines facing supply instability, as they are national policy tasks. However, she stressed the need to build social consensus given strong opposit
- Policy
- Reimb expanded for Opdivo, Yervoy…set for Vyloy, Padcev
- by Jung, Heung-Jun Oct 31, 2025 06:11am
- BMS Korea’s Yervoy (ipilimumab) and Ono Pharmaceutical Korea’s Opdivo (nivolumab) passed their first hurdle toward expanding reimbursement in Korea. Meanwhile, Janssen Korea’s Tecvayli (teclistamab), Pfizer Korea’s Elrexfio (elranatamab), and Astellas Korea’s Padcev (enfortumab vedotin) and Vyloy (zolbetuximab) had their reimbursement st
- Policy
- K-API·listed essential medicine to get preferential price
- by Lee, Jeong-Hwan Oct 30, 2025 06:11am
- The Ministry of Health and Welfare (MOHW) has decided to expand eligibility for the 68% preferential drug price for National Essential Medicines that use domestic active pharmaceutical ingredients (API). The MOHW aims to foster the domestic industry by incentivizing the use of domestically sourced API. The MOHW is shifting its initial sta
- Policy
- Jardiance generics listed late October…but not CKD
- by Jung, Heung-Jun Oct 30, 2025 06:09am
- As the substance patent for Jardiance expired on October 24, a total of 235 products&8212;both single-agent and combination formulations&8212;were simultaneously added to Korea’s reimbursement list. However, Chong Kun Dang chose a different approach, applying for sequential listing of its single-agent and combination products. The comp
- Policy
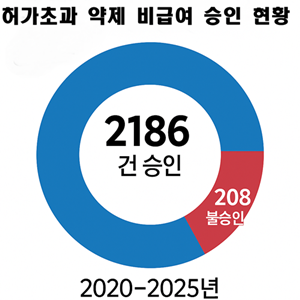
- Non-reimbursed approvals of off-label drugs
- by Jung, Heung-Jun Oct 29, 2025 06:10am
- The number of approvals for non-reimbursed use of drugs beyond their authorized indications (off-label) has been more than 10 times the number of rejections over the past six years. While 208 applications for off-label use were rejected, 2,186 were approved, often because the benefits outweighed the risks. On October 27, the Health Ins