- Policy
- COVID-19 Vaccine Damage Compensation Act passes NA
- by Lee, Jeong-Hwan Apr 4, 2025 05:58am
- A special law that compensates and supports patients who have suffered damage after COVID-19 vaccination was passed at the plenary session of the National Assembly on the afternoon of the 2nd. Also, a partial amendment to the Framework Act on Health and Medical Services, which includes the establishment of a Health and Medical Manforce Pl
- Policy
- First RSV vaccine Arexvy to soon be released in Korea
- by Lee, Hye-Kyung Apr 1, 2025 05:52am
- With the release date of the first RSV (respiratory syncytial virus) vaccine in Korea confirmed the MFDS has also set a fee for its national lot release approval. GSK Korea announced that it will launch the RSV-LRTD vaccine ‘Arexvy’ in May. Once the pharmaceutical company releases the vaccine, full-scale vaccination is expected to becom
- Policy
- KPBMA proposes 'drug shortages·API·AI new drug' budget
- by Lee, Jeong-Hwan Apr 1, 2025 05:52am
- Amid both ruling and opposition parties discussing the allocation of a supplementary budget plan, the Korean pharmaceutical industry has begun requesting a supplementary budget for two key initiatives: the "Production Support Business for Drugs with Supply Instability" and the "Direct Support Business for the Production of Active Pharmaceutic
- Policy
- Advanced biodrugs subject to reimb eval before approval
- by Lee, Jeong-Hwan Mar 31, 2025 05:59am
- 'Advanced biopharmaceuticals' will be added to the group of drugs that can receive drug reimbursement evaluations before receiving the government's official marketing authorization. The revision comes into effect from April 1st. On the 28th, the MOHW announced this through a notice of the product groups subject to the approval - insuranc
- Policy
- "Will maintain stability of essential·short supply drugs"
- by Lee, Jeong-Hwan Mar 28, 2025 06:39am
- The government will reportedly provide necessary administrative support for a stable supply of essential drugs and medications and improve the system so that innovative new drugs and new medical devices can quickly introduced into medical practices. Preferential pricing for national essential drugs that use domestically sourced raw materi
- Policy
- "Regulatory hurdle eliminated to simply refund-type RSA"
- by Lee, Jeong-Hwan Mar 28, 2025 06:37am
- The government saw eliminating regulatory hurdle as a success in exempting the efficacy·cost-effectiveness evaluation procedure during the 'third-contract termination evaluation' for pharmaceuticals on the basic refund-type RSA for over 10 years. Even drugs that have been subject to fines or other administrative sanctions can still have
- Policy
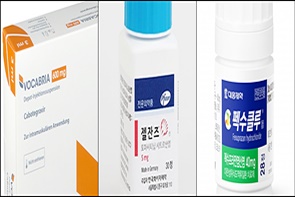
- Vocabria·Rekambys newly listed…expanded reimb for Xeljanz
- by Lee, Jeong-Hwan Mar 26, 2025 06:00am
- The HIV treatments Vocabria (cabotegravir) and Rekambys (rilpivirine) will be newly added to the National Health Insurance reimbursement list next month. The scope of reimbursement will be expanded for Pfizer's autoimmune diseases treatment Xeljanz (tofacitinib), Novartis' Cosentyx (secukinumab), and Daewoong's erosive gastroesophageal ref
- Policy
- New law proposed for the cancer and rare disease fund
- by Lee, Jeong-Hwan Mar 25, 2025 05:54am
- A bill to establish a new fund for cancer and rare diseases to strengthen patient access to ultra-high-priced drugs has been proposed to the National Assembly. The fund will be raised through transfers and deposits from other funds, such as the lottery fund. On the 24th, National Assembly member Myeong-ok Seo (People Power Party), a me
- Policy
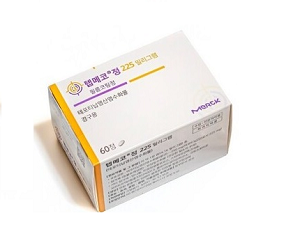
- Tepmetko, Tevimbra granted reimbursement in Korea
- by Lee, Tak-Sun Mar 24, 2025 05:52am
- New anticancer drugs Tepmetko and Tevimbra will be included in the list of reimbursed drugs as of April 1 in Korea. In addition, the economic burden on the patients is expected to be significantly reduced as the co-insurance rate for abiraterone acetate drugs such as Zytiga has been reduced for the first-line treatment of castration-resis
- Policy
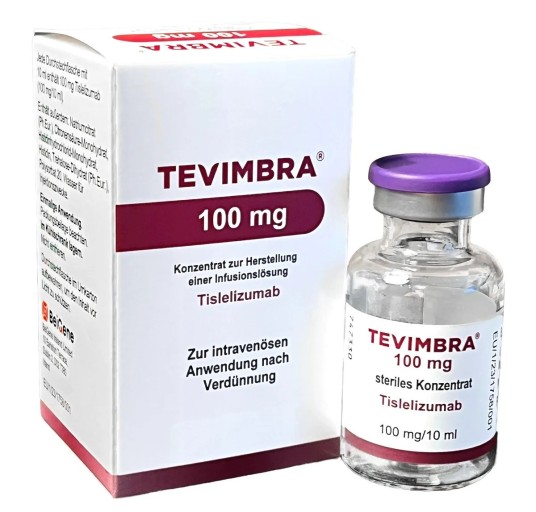
- How Tevimbra was reimbursed in Korea first
- by Lee, Tak-Sun Mar 24, 2025 05:52am
- BeiGene Korea's Tevimbra will be reimbursed in Korea from next month as a second-line treatment for esophageal squamous cell carcinoma, a type of esophageal cancer. It is the first immuno-oncology drug to be covered for esophageal cancer. In particular, the drug is drawing attention as it was covered in South Korea before the A8 countries