- Policy
- New drug approval fees ₩410mil in KOR
- by Lee, Hye-Kyung Aug 13, 2025 06:07am
- Since the new drug approval fee was significantly increased to KRW 410 million starting this year, a total of 14 new products containing 10 ingredients have been submitted for approval. Although the specific product names cannot be disclosed, dedicated teams have been formed for 6 chemical drug substances and four biopharmaceutical substances, a
- Policy
- Smokers at 54.5 times higher risk of SCLC than non-smokers
- by Lee, Tak-Sun Aug 12, 2025 06:13am
- A study has found that 30-year smokers have a 54.5 times higher risk of developing small cell lung cancer than non-smokers. The National Health Insurance Service (NHIS) Health Insurance Policy Research Institute announced on the 11th the results of a comparative analysis of cancer incidence risk and attributable risk by cancer type among indi
- Policy
- Will RSV vaccines be included in the NIP?
- by Whang, byung-woo Aug 8, 2025 06:03am
- With the RSV (respiratory syncytial virus) vaccine being released in Korea this year, interest in its inclusion in the National Immunization Program (NIP) has been gaining attention. According to the National Assembly's legislative information system, on the 6th, Rep. Yong-ki Jeon of the Democratic Party of Korea introduced a bill titled “Pa
- Policy
- Gov’t sets criteria for drug shortage prevention
- by Lee, Hye-Kyung Aug 7, 2025 06:06am
- The government will improve the criteria to raise the prices of drugs essential for patient treatment. Specifically, it will establish detailed evaluation criteria for price adjustment requests submitted by pharmaceutical companies that deem the current insurance price ceiling unreasonable. Prime Minister Min-seok Kim chaired the 7th Bioh
- Policy
- H1 Pharma exports amounted to $5.38B
- by Lee, Hye-Kyung Aug 7, 2025 06:05am
- In the first half of this year, healthcare industry exports increased by 13.2% compared to the same period last year, reaching $13.79 billion, an all-time high for a half-year period. By sector, exports were led by cosmetics at $5.51 billion (+14.9%), pharmaceuticals at $5.38 billion (+20.5%), and medical devices at $2.91 billion (△0.6%).
- Policy
- Faslodex’s price stays as is…supply instability concerns
- by Lee, Tak-Sun Aug 5, 2025 06:08am
- The breast cancer drug Faslodex (fulvestrant, AZ Korea), was facing a price cut due to the expiry of its premium pricing period, but this was prevented by the pharmaceutical company's request for a stay of execution. On the 31st of last month, the MOHW announced that the court had temporarily suspended the execution of the disposition to
- Policy
- MFDS clarifies support for advanced biopharmaceuticals
- by Lee, Hye-Kyung Aug 4, 2025 05:53am
- With the Ministry of Food and Drug Safety raising the approval and review fees for advanced biopharmaceuticals by up to KRW 410 million from January 1 this year, it has clarified the criteria for those eligible for fee reductions. In addition, detailed administrative disposition standards have been set for cases where human cell managers and
- Policy
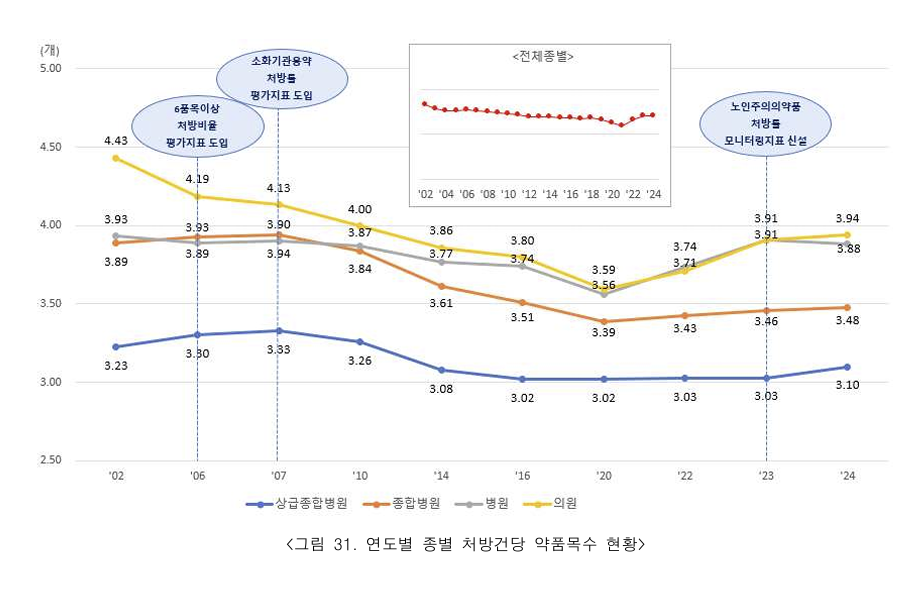
- ENT and pediatric departments prescribe the most drugs
- by Lee, Hye-Kyung Aug 1, 2025 06:16am
- Last year, medical institutions prescribed an average of 3.85 drugs per prescription. Outpatient clinics prescribed the most at 3.94, followed by hospitals at 3.88, general hospitals at 3.48, and tertiary hospitals at 3.10. The percentage of medical institutions prescribing 6 or more drugs increased by 0.79% from 18.34% in the previous year t
- Policy
- PPP also proposes free HPV vaccination for ppl under 26
- by Lee, Jeong-Hwan Aug 1, 2025 06:16am
- Following the ruling party, the main opposition party, the People Power Party, also submitted a bill to the National Assembly to provide free HPV vaccines to all citizens aged 26 and under, regardless of gender. The People Power Party’s bill also included provisions to expand the scope of free influenza vaccinations beyond the current co
- Policy
- MOHW seeks measures to attract domestic investment
- by Lee, Jeong-Hwan Jul 31, 2025 06:15am
- The government is seeking ways to encourage multinational pharmaceutical companies to strengthen domestic investment, including the establishment of new pharmaceutical production plants in Korea. However, international trade issues and other obstacles remain to be overcome to attract domestic investment from multinational pharmaceutical co