- Company
- Co-promotion discussed for the diabetes drug Mounjaro
- by Kim, Jin-Gu Aug 27, 2025 06:07am
- The possibility of a co-promotion partnership between Eli Lilly and Korean pharmaceutical companies for 'Mounjaro,' the treatment of diabetes and obesity, continues to be discussed. While Lilly Korea officially maintains a 'direct sales' policy, it is reported that they are also conducting undiscloed discussions for potential partnerships.
- Company
- CKD aims at developing next-generation·new anti-cancer drug
- by Son, Hyung Min Aug 26, 2025 06:06am
- Chong Kun Dang is speeding up investment in the new anti-cancer drug sector. The company aims to commercialize anti-cancer drugs by advancing its in-house pipelines for Antibody-Drug Conjugates (ADCs) and Cell and Gene Therapies (CGTs). Additionally, Chong Kun Dang is securing domestic rights for new drugs, including Chimeric Antigen Receptor T-
- Company
- Expanded indication for Kisqali in early breast cancer
- by Son, Hyung Min Aug 26, 2025 06:05am
- On August 22, Novartis Korea (CEO and President Byeong-jae Yoo) announced that its CDK 4/6 inhibitor, Kisqali, was approved for a new indication from the Ministry of Food and Drug Safety for use as an adjuvant therapy in patients with HR+ (hormone receptor-positive)/HER2- (human epidermal growth factor receptor 2-negative) Stage II and III e
- Company
- Will companies pass Korea due to Trump’s drug price reform?
- by Moon, sung-ho Aug 26, 2025 06:05am
- Since Trump took office, the US has designated the pharmaceutical industry as its core strategic industry and is working to reshape the industry with the US at its center. The goal is to significantly lower drug prices in the U.S., the world's largest pharmaceutical market. However, the “Most-Favored-Nation Pricing (MFN)” policy recently a
- Company
- Expanded competitiveness of K-Bio immunotherapies
- by Son, Hyung Min Aug 26, 2025 06:05am
- Korean pharmaceutical and biotech companies are entering the development of immunotherapies, increasing the possibility of 'big deals' with global pharmaceutical companies. As competition for new drugs targeting next-generation immune checkpoint proteins intensifies, Aprogen has begun developing VISTA targets by securing an antibody candidate fr
- Company
- Jaypirca may be prescribed at general hospitals in Korea
- by Eo, Yun-Ho Aug 26, 2025 06:04am
- The BTK inhibitor Jaypirca may now be prescribed in general hospitals in Korea. According to industry sources on the 22nd, Lilly Korea’s relapsed and refractory mantle cell lymphoma treatment Jaypirca (pirtobrutinib) has passed review of Drug Committees (DCs) at medical institutions in Korea, including Samsung Medical Center, Pusan Unive
- Company
- 'New treatment opportunities rise for acquired hemophilia A'
- by Whang, byung-woo Aug 25, 2025 06:06am
- There is an extremely rare bleeding disorder that affects one in every 100,000 people. It is acquired hemophilia A, a condition in which healthy individuals experience unexplained bruising and severe bleeding throughout the body. Until recently, there were limited treatment options available for emergencies, causing significant challenges for
- Company
- Lilly seeks Mounjaro’s reimbursement for diabetes in Korea
- by Eo, Yun-Ho Aug 22, 2025 06:08am
- ‘Mounjaro,’ which is causing a roar in the field of obesity treatment, is seeking inclusion in insurance reimbursement for its diabetes indication. According to Dailpharm coverage, Eli Lilly Korea has submitted a reimbursement application for its Mounjaro (tirzepatide), a dual GIP/GLP-1 receptor agonist, and is proceeding with the neces
- Company
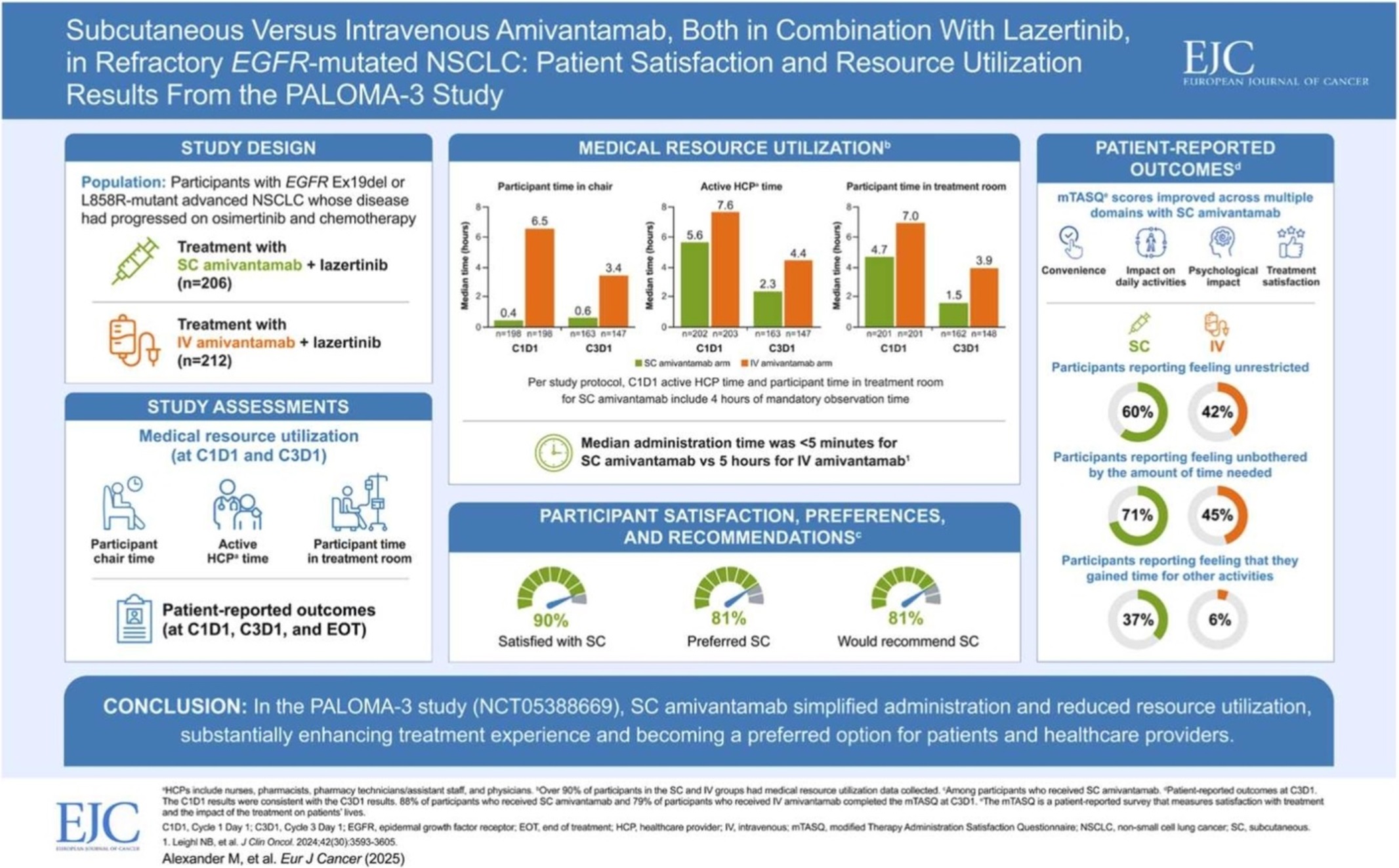
- Leclaza at global crossroads 1 year into FDA approval
- by Moon, sung-ho Aug 22, 2025 06:06am
- One year has passed since Leclaza (lazertinib) received FDA approval in combination with Johnson & Johnson’s Rybrevant (amivantamab). Expanding its influence beyond the United States to Europe and Asia, it has emerged as a global treatment option both in Korea and abroad. In the first half of this year, it recorded an overall survival rate (
- Company
- Nubeqa gains flexibility with indication expansion
- by Whang, byung-woo Aug 21, 2025 06:06am
- The influence of Nubeqa (darolutamide) is rising in the market with the company expanding its indication as a treatment for metastatic hormone-sensitive prostate cancer (mHSPC). Experts believe that the drug may settle as a flexible treatment option in Korea’s market as it has broadened its path as a personalized treatment. With the approval