- Policy
- “MOHW neglected 10000 practices by 56 suspended doctors”
- by Kim, Jung-Ju Nov 4, 2019 03:11pm
- Sources report tens of doctors, Korean medicine doctors and dentists with suspended licenses have practiced over 11,000 medical services and claimed over 800 million won of insurance reimbursement. The Board of Audit and Inspection of Korea (BAI) notified the details the health authority and ordered to take a corrective action on its n
- Company
- Companies ready litigation against valsartan damage claim
- by Chon, Seung-Hyun Nov 4, 2019 03:10pm
- The second deadline for valsartan damage indemnity payment the health authority is claiming from pharmaceutical companies has been passed. Most of companies are refusing to pay and preparing for litigation. 34 companies have decided to take a joint legal action and designated a large law firm to represent them. According to an industry insid
- Policy
- Reviewing 1 year of Rare Disease Support Scheme
- by Eo, Yun-Ho Nov 4, 2019 03:09pm
- Now is the time to take a greater interest in and discover issues of rare disease treatment coverage. The Moon Care’s ‘reimbursement expansion on the non-reimbursed’ would mean nothing for rare disease without ‘awareness’ of the nature. The reason behind tough rare disease treatment reimbursement listing seems complicated, but it
- Company
- Pfizer's 50 years of ‘changes and innovation’ in Korea
- by Byun Kyung A Nov 4, 2019 08:13am
- Half a century in Korean pharmaceutical industry, Pfizer Korea seems to have mastered ‘how to win’ in the Korean market. Pfizer took its first step into the Korean market as a joint corporation with Joongang Pharmaceutical in 1962 and founded Pfizer Korea in 1969. Except for a couple of times, Pfizer Korea since then consistently rec
- Policy
- First year of Rare Disease Support Scheme
- by Eo, Yun-Ho Nov 4, 2019 08:13am
- The biggest issue of the policy is ‘lack of interest’. ‘Rare disease’ is not a specific categorization, but rather it is designated based on frequency of diagnosis. Korea defines ‘rare disease’ as a disease diagnosed to less than 20,000 people. With small patient size and lack of drug, these diseases are in dire need of new drug. B
- InterView
- MFDS nizatidine investigation on NDMA still ongoing
- by Byun Kyung A Nov 1, 2019 04:45pm
- For the first time, Ministry of Food and Drug Safety (MFDS) officially announced its investigation on possibility of finding NDMA carcinogen in nizatidine, a structurally similar substance to ranitidine. Director Kim Nam-Soo of Pharmaceutical Management Division at MFDS convened a press conference on Oct. 29 and shared the news. The Pha
- Policy
- Industry burdened but accepting of mandatory DSUR
- by Byun Kyung A Oct 31, 2019 09:59am
- Pharmaceutical industry initially complained of administrative strain when the government issued a notice on enforcing mandatory drug safety update report (DSUR)’, as advised by the National Assembly. But the industry also agrees with Korean government’s intention to enforce the pre-marketing regulation, which now has become a global tren
- Product
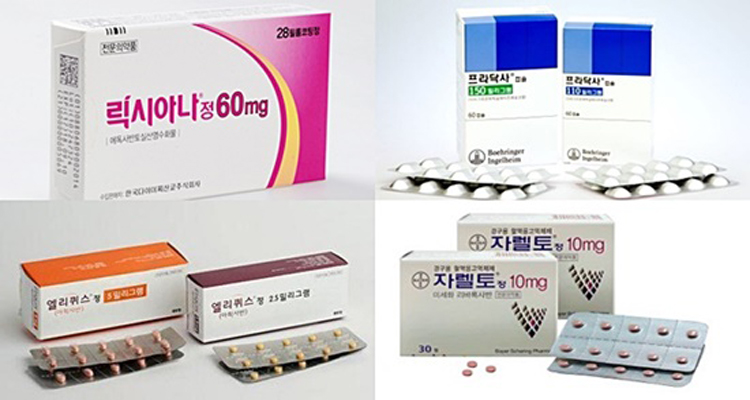
- Lixiana joins NOAC market race late but takes the lead
- by Byun Kyung A Oct 31, 2019 09:59am
- A follow-on drug in the non-vitamin K antagonist oral anticoagulant (NOAC) market, Lixiana, continues to lead the market. The treatment took the lead from Xarelto in last January for the first time, and it has been the most prescribed drug in the market for nine consecutive months. Pfizer and Bristol-Myers Squibb’s Eliquis had to face a Ko
- Policy
- Weighted pricing benefit abused as permanent price booster
- by Byun Kyung A Oct 31, 2019 09:58am
- The government evaluated the current weighted drug pricing system has been digressed as a permanent pricing benefit. Apparently, 96 percent of items have maintained the benefitted pricing for more than three years, and the authority sees that such practice has distanced itself from the initial objective to secure stable supply. Ministry
- Product
- Maviret takes up 83% of HCV treatment market share
- by Byun Kyung A Oct 31, 2019 09:41am
- Abbvie broke through the hepatitis C virus (HCV) treatment market and dominated 83 percent of oral outpatient prescription market only with its pan-genotype HCV treatment, Maviret, launched last year. Gilead once dominated the market with two items, but their prescription ratio shriveled down to 12 percent. On Oct. 30, a pharmaceutic