- Policy
- Industry expects improved pricing measures by new gov't
- by Lee, Jeong-Hwan Jun 23, 2025 06:01am
- The domestic pharmaceutical industry is closely monitoring the direction of the Lee Jae-Myung administration's preferential drug pricing policy. The industry is most interested in when and how the presidential election pledges, including specific implementation plans for the new drug R&D-linked preferential drug pricing policy and the in
- Policy
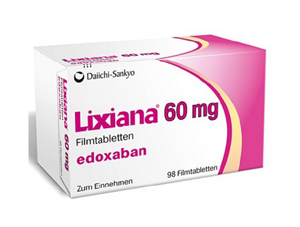
- Generic 'Lixiana' is now in 'active' development in KOR
- by Lee, Hye-Kyung Jun 23, 2025 06:01am
- Korean pharmaceutical companies are actively working on plans to launch their generic version of 'Lixiana Tab 60 mg (edoxaban tosylate hydrate),' a Direct Oral Anti-Coagulant (DOAC) with patent expiry set to November of next year. The Ministry of Food and Drug Safety (MFDS) recently approved Korean Drug Co's application for a bioequivalenc
- Policy
- Handok and JW’s new imported drugs reimbursed from July
- by Lee, Tak-Sun Jun 23, 2025 06:00am
- In July, four new drugs, including an oral drug for paroxysmal nocturnal hemoglobinuria (PNH), a new biological agent for severe atopic dermatitis, and a new drug for immune thrombocytopenia (ITP), will be reimbursed by health insurance and be available with coverage for patients. On the 19th, the MOHW announced the establishment of reim
- Policy
- Rapid adjustment of insurance prices for rare drugs granted
- by Lee, Hye-Kyung Jun 20, 2025 06:08am
- The insurance price ceiling adjustment term for insured drugs supplied by the Korea Orphan Drug Center (KOEDC) is expected to be significantly reduced from the initial 8-9 months to around 4 months. Previously, it took at least 8-9 months to adjust the price cap, raising concerns about supply disruptions and financial losses for the cente
- Policy
- SGLT2 Jardiance likely to receive expanded reimb for CKD
- by Lee, Tak-Sun Jun 20, 2025 06:06am
- The SGLT-2 inhibitor Jardiance (empagliflozin, Boehringer Ingelheim) is expected to be reimbursed for the treatment of chronic kidney disease (CKD). This drug is currently reimbursed for the treatment of diabetes and chronic heart failure. If expanded reimbursement is approved for CKD, the volume of usage is likely to be increased. Further
- Policy
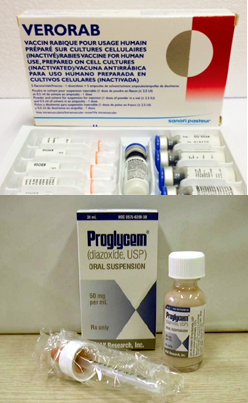
- Growing role of the Korea Orphan & Essential Drug Center
- by Lee, Hye-Kyung Jun 19, 2025 06:03am
- "The emergency import of essential medicines through the Korea Orphan & Essential Drug Center (KOEDC) will be expanded, and support for pharmaceutical companies producing domestic products will be planned." President Lee Jae-myung made this pledge on his Facebook page on May 28, during his campaign for the presidency. President Lee emphasized
- Policy
- Petition filed urging expedited reimbursement of Livmarli
- by Lee, Jeong-Hwan Jun 17, 2025 06:00am
- A national petition has been filed to urge for the health insurance reimbursement of Livmarli (maralixibat), which is used to treat pruritus (itching) in patients with Alagille syndrome. The petition period runs until the 12th of next month, with over 1,600 signatures signed as of the 16th. Alagille syndrome is a condition in which the in
- Policy
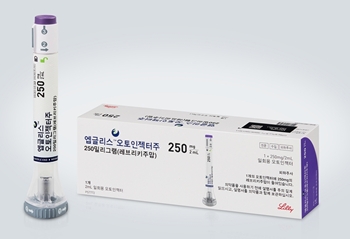
- Atopic dermatitis drug Ebglyss reimbursed from next month
- by Lee, Tak-Sun Jun 16, 2025 06:03am
- With pricing negotiations between the National Health Insurance Service and the manufacturer of the new atopic dermatitis drug ‘Ebglyss Autoinjector (Lebrikizumab, Lilly Korea)’ complete, the drug is expected to be listed for reimbursement next month in Korea. Once listed, the number of biological agents available for atopic dermatitis
- Policy
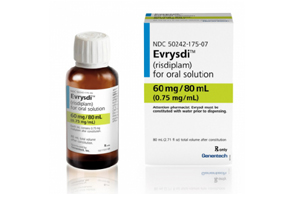
- Roche’s SMA drug Evrysdi Tab approved in Korea
- by Lee, Hye-Kyung Jun 16, 2025 06:03am
- The table formulation of Roche's blockbuster drug for spinal muscular atrophy (SMA), Evrysdi (risdiplam), has received marketing authorization in Korea. On the 13th, the Ministry of Food and Drug Safety approved Roche Korea's ‘Evrysdi 5 mg (risdiplam).’ With the approval, SMA patients in Korea may now be prescribed the 5 mg tablet fo
- Policy
- Ruling party proposes bill for free HPV vaccination
- by Lee, Jeong-Hwan Jun 13, 2025 06:02am
- The Democratic Party of Korea has submitted a bill to the National Assembly to significantly expand the scope of Korea’s National Immunization Program for human papillomavirus (HPV) vaccines. The bill proposes to provide free HPV vaccinations to all males and females aged 12 to 26, regardless of gender or income level. This legislatio