- Policy
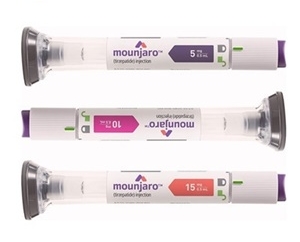
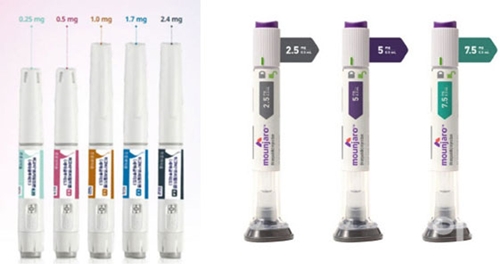
- Ozempic nears reimb approval…next is Mounjaro
- by Jung, Heung-Jun Oct 13, 2025 06:02am
- With Novo Nordisk’s GLP-1 injectable Ozempic (semaglutide) deemed adequate for reimbursement for diabetes, attention is now turning to whether Eli Lilly’s Mounjaro (tirzepatide) will be reviewed next by the Health Insurance Review and Assessment Service (HIRA). At the same time, voices are growing in favor of extending insurance coverag
- Policy
- 'Low-price purchase incentive ineffective and detrimental'
- by Lee, Jeong-Hwan Oct 10, 2025 06:05am
- There is growing criticism that the government’s low-price drug purchase incentive policy should be overhauled due to its structural contradictions and low effectiveness. The low-price purchase incentive, which is linked to the market-based actual transaction price reduction system, focuses on price rather than quality. From the pharmace
- Policy
- GLP-1 drug Ozempic passes reimbursement review
- by Jung, Heung-Jun Oct 10, 2025 06:05am
- Novo Nordisk’s GLP-1 receptor agonist Ozempic (semaglutide) has passed review by the Health Insurance Review and Assessment Service (HIRA)’s Drug Reimbursement Evaluation Committee, which acknowledged the drug as adequate for reimbursement. Ozempic contains the same active ingredient as the obesity drug Wegovy, but is indicated for diabetes.
- Policy
- "Why are pharma developing salt changes called innovative?"
- by Lee, Jeong-Hwan Oct 1, 2025 06:10am
- "There are no developed countries that provide public funds and National Health Insurance resources to pharmaceutical companies for simply changing salt formation. South Korea is the only country in the world that recognizes incrementally modified drugs as having the value of innovative new drugs. (If we want to call incrementally modified d
- Policy
- First lot of returning nasal spray flu vaccine approved
- by Lee, Tak-Sun Sep 30, 2025 06:12am
- The nasal spray flu vaccine ‘FluMist Intranasal Spray (AstraZeneca Korea)’ has received shipment approval from the Ministry of Food and Drug Safety (MFDS) and is preparing for sales. This product was previously sold by GC Biopharma, but it did not gain significant popularity at the time. With expected demand from children who dislike inj
- Policy
- MFDS, GLP-1 obesity drug + diabetes med combo hypoglycemia↑
- by Lee, Tak-Sun Sep 30, 2025 06:11am
- The Ministry of Food and Drug Safety (MFDS) announced that patients with diabetes using GLP-1 obesity treatments, which have recently become popular, must consult with their healthcare provider, as the co-administration of these drugs with diabetes medication significantly increases the risk of hypoglycemia. The MFDS also stressed that th
- Policy
- MoHW and NHIS agree on need to expand expedited listing
- by Jung, Heung-Jun Sep 29, 2025 06:08am
- The Ministry of Health and Welfare (MoHW) expressed consensus on the need to expand fast-track reimbursement to improve access to treatments for rare and severe diseases. As the government agenda already includes system improvements to reflect the innovative value of new drugs, the ministry stated its intent to support these reforms. On th
- Policy
- “Cut prices of generic drugs across the board”
- by Jung, Heung-Jun Sep 29, 2025 06:07am
- A proposal has been made to establish reimbursement priority for treatments targeting rare/serious diseases versus mild conditions, thereby expanding access to new drugs. There were also calls to reform the distorted pharmaceutical budget structure by cutting generic prices across the board and canceling approvals for products lacking bioequi
- Policy
- HIRA, "Will enhance the monitoring of rare disease drugs"
- by Jung, Heung-Jun Sep 26, 2025 06:12am
- With the rapid increase in newly emerging treatments for rare and severe diseases, there have been suggestions for establishing sustainable systems, such as strengthening post-market management and creating a separate dedicated fund. It was explained that monitoring uncertain evidence resulting from expedited approvals and improving patient
- Policy
- Legislation of restricted INN Prescriptions gain momentum
- by Lee, Jeong-Hwan Sep 26, 2025 06:11am
- With the ruling party accelerating legislation of “restricted international nonproprietary name (INN) prescriptions,” which was one of President Jae-myung Lee’s campaign pledges, the medical community is showing visible unease. Democratic Party of Korea lawmakers In-soon Nam, Young-seok Seo, Yoon Kim, and Jong-tae Jang, along with Rebu