- Policy
- [Reporter's View] A separate fund for new orphan drugs
- by Lee, Jeong-Hwan Jul 18, 2025 06:36am
- Will the 'establishment of a separate fund,' considered one of the ways to improve patient access to expensive, rare, and intractable disease drugs, become even more difficult with the inauguration of the Lee Jae Myung administration? Jung Eun-kyung, the candidate for Minister of Health and Welfare (MOHW), who is facing a parliamentary co
- Policy
- Will the external reference pricing reevals gain momentum?
- by Lee, Jeong-Hwan Jul 18, 2025 06:35am
- Eun-Kyung Jeong, nominee for Minister of Health and Welfare, said that “it is important to manage drug prices at an appropriate level given the limited health insurance resources” regarding the post-marketing price review system to lower generic drug prices through “external reference pricing evaluations,” suggesting the possibility of i
- Policy
- Jung Eun-kyung views "orphan disease fund establishment"
- by Lee, Jeong-Hwan Jul 18, 2025 06:35am
- Jung Eun-kyung, the candidate for Minister of Health and Welfare (MOHW), has expressed skepticism regarding the establishment of a separate fund to improve the National Health Insurance reimbursement rate for treatments for expensive, rare (orphan), and severe diseases. The rationale is that if the required financial scale for reimbursing
- Company
- Denmark's non-profit foundation's virtuous governance cycle
- by Jul 18, 2025 06:35am
- In 1922, Dr. August Krogh paid just $1 to introduce insulin production technology from the University of Toronto in Canada. At the time, he made two promises to the University of Toronto research team: to make insulin available to all patients at an affordable price and to use insulin for public healthcare rather than for commercial gain.
- Opinion
- [Reporter's View] Bio policy should remain consistent
- by Kim, Jin-Gu Jul 17, 2025 06:13am
- In November 2024, the Yoon Suk Yeol government officially announced the launch of the National Bio Committee. The government aimed to operate a 'presidential' governance body in a preparatory response to the global bioeconomy era. Considering that the previously established 'Bio-health Innovative Committee' was chaired by the Prime Minister,
- Company
- Pharmaceutical exports to U.S. have surged by 46%
- by Kim, Jin-Gu Jul 17, 2025 06:13am
- Korea-made pharmaceutical exports to the United States have surged. In the first half of this year, exports to the U.S. amounted to approximately KRW 1.53 trillion, a 46% increase compared to the same period last year. Notably, exports to the U.S. saw an exceptional surge in June. The export in June alone was almost the total of the preceding fo
- Policy
- When will Keytruda’s reimb be reviewed by DREC?
- by Lee, Tak-Sun Jul 17, 2025 06:13am
- Attention is focused on when Keytruda, for which reimbursement standards were set for 11 indications in February, will be submitted to the Drug Reimbursement Evaluation Committee (DREC) for review. This is because, although the reimbursement standards were established by the Health Insurance Review and Assessment Service's Cancer Review C
- Company
- How Denmark gave birth to the golden goose Wegovy
- by Cha, Jihyun Jul 17, 2025 06:13am
- Novo Nordisk, the Danish company that developed the obesity treatment Wegovy that shook the world, rose to the top in Europe in terms of market capitalization in 2023. It surpassed France's luxury goods group LVMH, which had held the top spot in the European stock market for over two years. At the time, Novo Nordisk’s market value was appr
- Company
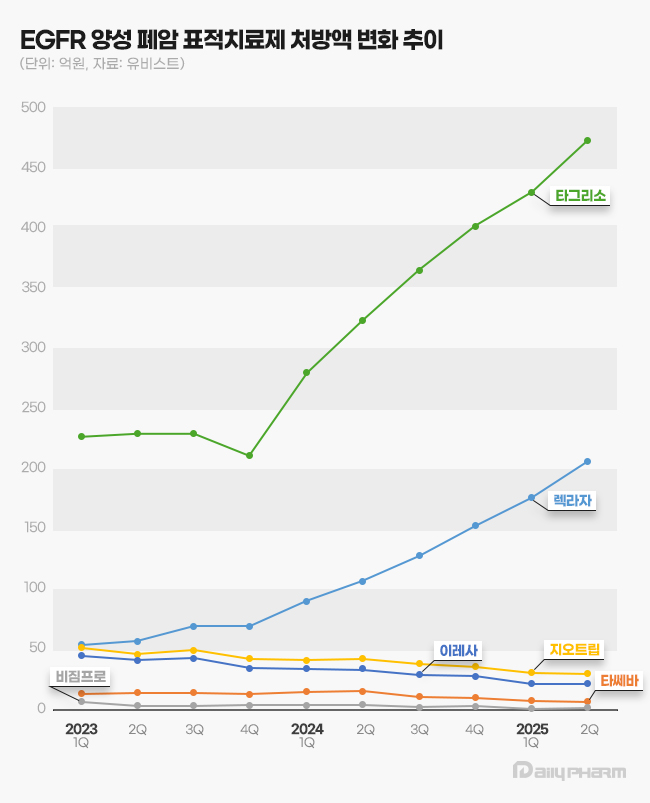
- Oral lung cancer drug market race
- by Son, Hyung Min Jul 16, 2025 06:10am
- Late entrants, such as Leclaza and Lorviqua, are expanding their presence in the Korean lung cancer targeted therapy market, continuing rapid growth. While prescription sales for some EGFR and ALK-positive non-small cell lung cancer (NSCLC) treatments are stagnating or declining, newer drugs in these categories are showing a clear upward trend,
- Opinion
- [Reporter's View] Gov’t cooperation leads to a price cut?
- by Eo, Yun-Ho Jul 16, 2025 06:10am
- There are times when cooperation ends up causing losses. In an ironic twist, pharmaceutical companies that participated in the government's infertility support program have now found their products caught in the net of the Price-Volume Agreement (PVA) system, leaving them little choice but to face rapid drug price cuts. The government's i