- Company
- New pneumococcal vaccine expected to be launched
- by Whang, byung-woo Jun 19, 2025 06:04am
- As 'Capvaxive,' a 21-valent pneumococcal conjugate vaccine (PCV21) developed by MSD, is anticipated to receive marketing authorization in South Korea, competition in the market is likely to heat up. According to pharmaceutical industry sources, MSD has filed with the Ministry of Food and Drug Safety (MFDS) for marketing authorization of Ca
- Company
- K-Bios face string of clinical failures in H1
- by Son, Hyung Min Jun 19, 2025 06:04am
- A series of clinical trial failures for new drug candidates under development by Korean biotech companies in the first half of the year have raised concerns about the feasibility of future technology exports. Orum Therapeutics halted a clinical trial due to safety concerns, while Genexine and Bridge Biotherapeutics both failed to demonstrate sta
- Company
- KOR-JPN jointly launches Healthcare Distribution Alliance
- by Son, Hyung Min Jun 19, 2025 06:03am
- Three pharmaceutical distribution companies in Korea and Japan have joined forces to launch the Healthcare Distribution Alliance to lead the domestic market by introducing advanced overseas models. The alliance aims to go beyond simple logistics agreements &8211; it seeks to build an innovative cooperation structure where companies can shar
- Policy
- Growing role of the Korea Orphan & Essential Drug Center
- by Lee, Hye-Kyung Jun 19, 2025 06:03am
- "The emergency import of essential medicines through the Korea Orphan & Essential Drug Center (KOEDC) will be expanded, and support for pharmaceutical companies producing domestic products will be planned." President Lee Jae-myung made this pledge on his Facebook page on May 28, during his campaign for the presidency. President Lee emphasized
- Opinion
- [Reporter's View] Telemedicine in a 5-sided tug-of-war
- by Lee, Jeong-Hwan Jun 19, 2025 06:02am
- With the election and inauguration of President Lee Jae-Myung, the Democratic Party of Korea, which successfully changed the administration, submitted a bill to revise the Medical Service Act that narrows the scope of telemedicine’s initial consultation in the current pilot program to the National Assembly. As a result, the eyes of the
- Company
- Takeda launches new drug 'Fruzaqla' in Korea
- by Whang, byung-woo Jun 18, 2025 10:28am
- Takeda Pharmaceutical Korea (CEO Kwang-kyu Park) announced on June 16 that the company has officially launched its 'Fruzaqla (fruquintinib)' in South Korea. Fruzaqla is the first new treatment for metastatic colorectal cancer. This drug selectively inhibits Vascular Endothelial Growth Factor Receptor (VEGFR)-1,2, and 3. It is expected
- Company
- Daiichi Sankyo exceeds ₩300B in sales…new drug drives
- by Son, Hyung Min Jun 18, 2025 06:01am
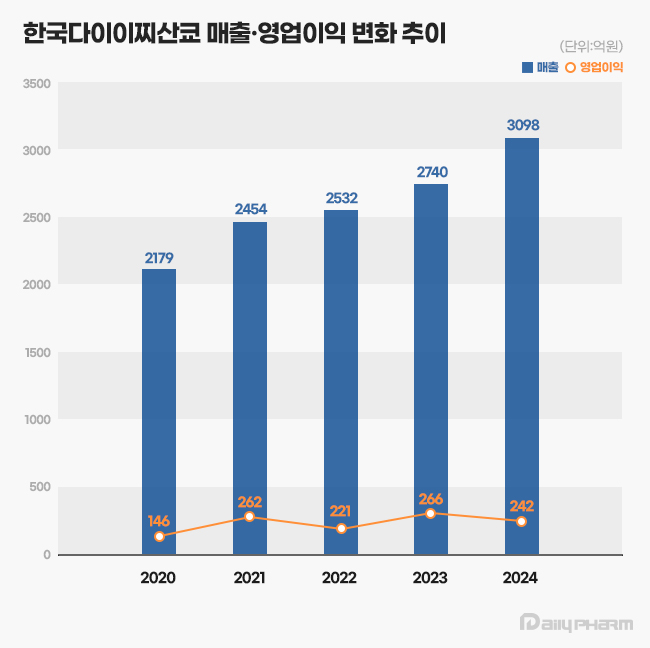
- Daiichi Sankyo Korea has exceeded KRW 300 billion in sales for the first time, led by its cardiovascular products, Antibody-Drug Conjugate (ADC), and new anticancer drugs. The company is successfully transitioning its portfolio towards new ADC drugs while maintaining robust growth from established cardiovascular products like Sevikar, Lixiana, a
- Opinion
- [Desk View] Disclosing drug reimb info must be done properly
- by Lee, Tak-Sun Jun 18, 2025 06:00am
- Due to concerns about the non-transparency of the selection process for medicinal product reimbursement, the Health Insurance Review & Assessment Service (HIRA) and the National Health Insurance Service (NHIS) are disclosing partial processes and candidates. HIRA discloses, through press releases, the review results from the Cancer Drug Re
- Company
- Zejula, a new standard ovarian cancer maintenance therapy
- by Whang, byung-woo Jun 18, 2025 06:00am
- Ovarian cancer is often diagnosed at an advanced stage due to the difficulty of early detection, and it is known for its high recurrence rate even after initial treatment. First-line maintenance therapy aimed at delaying recurrence as much as possible after surgery and chemotherapy became a key strategy that determines treatment outcomes for
- Company
- Adstiladrin receives orphan drug designation in Korea
- by Eo, Yun-Ho Jun 18, 2025 05:59am
- The new bladder cancer drug Adstiladrin has been designated as an orphan drug in Korea. The Ministry of Food and Drug Safety recently announced the news in a orphan drug designation announcement. The specific indication for designation is “treatment of BCG-refractory high-risk non-muscle-invasive bladder cancer (NMIBC) with carcinoma