- Company
- SK bioscience invests KRW 4.1B stake in a U.S. biotech
- by Kim, Jin-Gu Oct 10, 2024 05:51am
- SK bioscience announced on October 8th that the company has signed an agreement to acquire a stake in U.S. biotech Fina Biosolutions by investing USD 3 million (approximately KRW 4.1 billion). SK bioscience became the first and only strategic investor of Fina Biosolutions. According to the contract between both companies, the specific stake
- Company
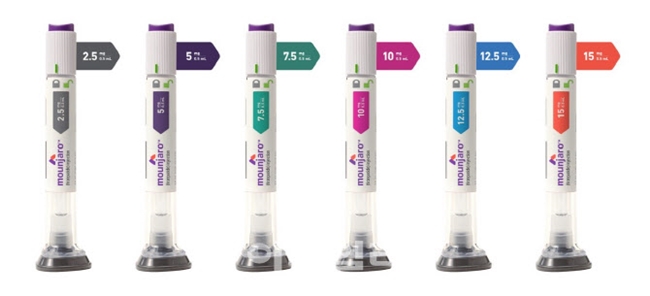
- Who will distribute Mounjaro in Korea?
- by Whang, byung-woo Oct 10, 2024 05:50am
- As Novo Nordisk's obesity drug Wegovy (semaglutide) is set to be released, the industry’s interest is on which domestic distributor Lilly will choose for its Mounjaro (tirzepatide). According to industry sources on the 8th, Boryung Pharmaceutical and Chong Kun Dang are in the running to become domestic distributors of Wegovy’s rival ob
- Policy
- "Preferential drug pricing excludes K-made new drug benefits
- by Lee, Jeong-Hwan Oct 10, 2024 05:50am
- The government's revised plan for the drug pricing system has been criticized for reverse discrimination against Korean pharmaceutical companies because it was primarily designed to favor multinational pharmaceutical companies. The criticism concerns the revised drug pricing system plan, announced by the Health Insurance Review and Assessment
- Opinion
- [Reporter's View]Industry expectations rise with new changes
- by Eo, Yun-Ho Oct 10, 2024 05:50am
- The criteria for innovative new drugs that are eligible for the flexible application of the ICER threshold and the measures for the expansion of the risk-sharing agreement (RSA) have been revealed. As always, the industry expressed a mix of expectations and concerns. The disclosed ‘Detailed evaluation criteria for new drugs etc. subject
- Policy
- Yoon Kim ‘40% of the generic drug price is a bubble’
- by Lee, Jeong-Hwan Oct 10, 2024 05:50am
- Kyoo-hong Cho, the Minister of Health and Welfare, positively responded to Democratic Party of Korea Representative Yoon Kim's request to lower the price of generic versions of patent-expired drugs within the year. This refers to a separate generic drug price cut rather than the foreign drug price referencing reevaluations, which the phar
- Company
- Wegovy's release on the 15th…competitor Mounjaro
- by Whang, byung-woo Oct 8, 2024 05:49am
- With the release of Wegovy, the next-generation obesity drug, set for the middle of this month (October), the release of its competitor Mounjaro is also gaining attention. Although Lilly has not yet announced a specific launch date for its Mounjaro, the company has opted to add another indication to its drug rather than apply for new drug app
- Company
- Ilaris can be prescribed at general hospitals in KOR
- by Eo, Yun-Ho Oct 8, 2024 05:49am
- Ilaris, which was listed for reimbursement 9 years after receiving marketing authorization in Korea, can now be prescribed at general hospitals in Korea. According to industry sources, Novartis Korea's hereditary recurrent fever syndrome drug Ilaris (canakinumab) has passed the drug committees (DCs) of Seoul National University Hospital,
- Policy
- Daewoong wins nod for first generic version of Ibrance tab
- by Lee, Tak-Sun Oct 8, 2024 05:49am
- Daewoong's first generic for Pfizer's breast cancer treatment, 'Ibrance Tab,' has been approved. Due to the successful patent nullification, the company received priority marketing authorization. The first generic can be launched in March 23rd, 2027, when the product patent expires. The Ministry of Food and Drug Safety (MFDS) has granted
- Company
- Eisai Korea signs Alzheimer's MOU with the Korean Dementia
- by Whang, byung-woo Oct 8, 2024 05:49am
- Eisai Korea announced on October 7th that it has signed a memorandum of understanding (MOU) with the Korean Dementia Association to utilize and manage 'JOY-ALZ,' a registry program for long-term follow-up survey platform of Alzheimer's disease prognosis. The MOU aims to advance Alzheimer's disease treatment and to establish safe Alzheime
- Company
- Galderma Korea appoints Jai Hyuk Lee as new GM
- by Whang, byung-woo Oct 8, 2024 05:48am
- Galderma Korea announced on the 7th that it has appointed Jake (Jai Hyuck) Lee, former head of the Aesthetics Business Unit, as its new General Manager, effective as of October 1. The new GM is a seasoned sales and marketing expert with more than 25 years of experience in the global pharmaceutical and aesthetics industries. Lee will succe