- Company
- Jardiance generics prepare for final launch in Korea
- by Kim, Jin-Gu Sep 30, 2024 05:47am
- Generic companies are in the midst of last-minute preparations to enter the market for the SGLT inhibitor diabetes drug Jardiance (empagliflozin), which is set to expire in just about half a year. With Forxiga’s market withdrawal expected to leave an annual sales gap of KRW 50 billion, the race to fill this gap is expected to intensify among
- Company
- New myelodysplastic syndrome drug 'Reblozyl' lands Big 5 DC
- by Eo, Yun-Ho Sep 30, 2024 05:46am
- The treatment for myelodysplastic syndrome (MDS), 'Reblozyl,' is available for prescription at tertiary general hospitals. Sources said that Bristol Myers Squibb (BMS) Pharmaceutical Korea's Reblozyl (luspatercept) has passed all drug committees (DC) of the 'Big 5' medical centers, including Samsung Medical Center, Seoul National Univers
- Company
- Phesgo can be prescribed in Korea with reimbursement
- by Eo, Yun-Ho Sep 30, 2024 05:46am
- Breast cancer biobetter ‘Phesgo’ can now be prescribed in general hospitals in Korea with insurance reimbursement. According to industry sources, Phesgo (pertuzumab/trastuzumab), which is a subcutaneous injection combination of Roche's Perjeta and Herceptin, has passed drug committees (DC) reviews of tertiary hospitals in Korea, includi
- Company
- K-Phama companies are targeting Southeast Asia
- by Heo, sung-kyu Sep 27, 2024 05:53am
- Korean pharmaceutical companies target Indonesia to establish a bridgehead for Southeast Asia market entry. Companies have invested in Indonesia by buidling manufacturing plants. The government and associations are also in support. According to pharmaceutical companies on September 20th, pharmaceutical companies, the government, and or
- Company
- 'Treatment options for psoriasis are evolving'
- by Son, Hyung Min Sep 27, 2024 05:53am
- “In the field of psoriasis, the development of many biologics has been largely abandoned due to the lack of convincing data showing similar efficacy to marketed drugs. On the other hand, in the field of oral drugs, interest in the TYK2 mechanism has increased upon the introduction of Sotyktu, increasing the industry’s R&D on such oral dr
- Company
- KRPIA expresses concerns over drug approval fee hike
- by Eo, Yun-Ho Sep 27, 2024 05:53am
- “The pharmaceutical industry is bound to feel burdened by the sudden decision. We hope that the new drug approval system will be adjusted through discussions with the industry.” The Korean Research-based Pharmaceutical Industry Association (KRPIA) expressed the pharmaceutical industry's stance regarding the amendment to the 'Fee Regulat
- Company
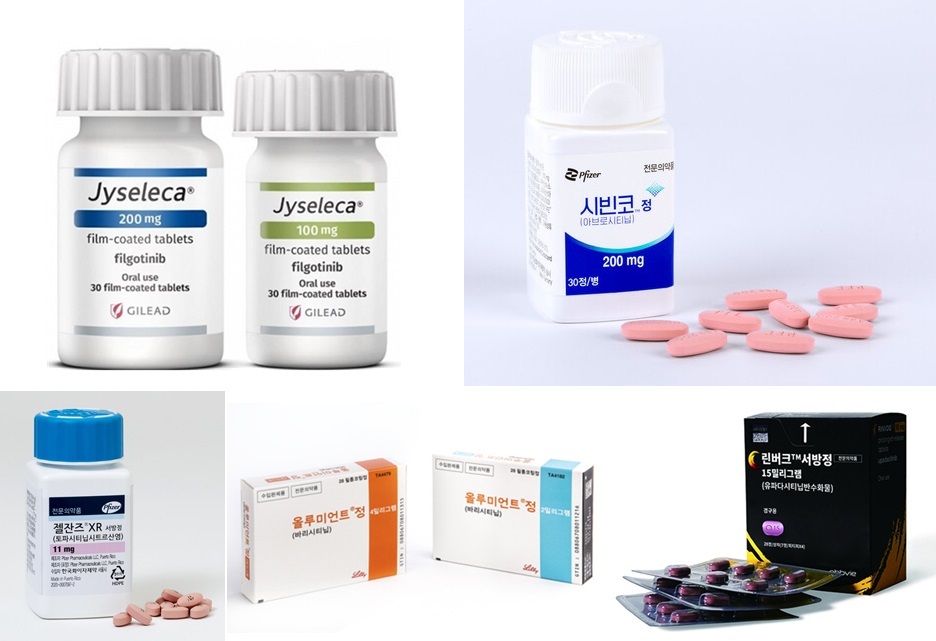
- Switching between JAKis for RA yet to be reimbursed
- by Eo, Yun-Ho Sep 27, 2024 05:52am
- The plan to allow insurance reimbursement when switching between JAK inhibitors in rheumatoid arthritis, which was expected to take effect in October, has been postponed. According to Dailypharm coverage, the health authorities have recently put on hold the notification of the proposed revision that allows switching between JAK inhibitors
- Company
- Minjung Jung to head Corporate Affairs at Sanofi KR, NZ, AU
- by Eo, Yun-Ho Sep 26, 2024 05:51am
- Sanofi’s Executive Director Minjung Jung (48) has been named head of Corporate Affairs for the Sanofi Group's 3 subsidiaries - Korea, New Zealand, and Australia. The appointment follows the appointment of Kyungeun Bae (53) as the General Manager of Pharma MCO South Korea and Australia/New Zealand & MCO Lead in the first half of last year
- Company
- "Bird flu: a new emerging pandemic risk factor"
- by Son, Hyung Min Sep 25, 2024 05:49am
- The industry is preparing against avian influenza, which can be transmitted from animal to human, as it is predicted to be the next pandemic risk factor. Experts suggest that an improved vaccine technology development and manufacturing system are required ahead of the emerging pandemic following COVID-19. CSL Seqirus Korea held a press co
- Company
- ‘Link the processes to improve access to orphan drugs'
- by Kim, Jin-Gu Sep 25, 2024 05:49am
- A claim has been raised that the approval, evaluation, and negotiation linkage system should be introduced to strengthen access to rare disease drugs. Also, the claim that the pharmacoeconomic evaluation system should be flexibly applied to rare disease drugs and that the scope of the risk-sharing agreement system be expanded was raised at the t