- Company
- Aftermath of Januvia's price cut…MSD-CKD compensation fight
- by Son, Hyung Min Jul 14, 2025 06:04am
- Regarding transaction for price difference compensation following the drug price reduction for the diabetes treatment Januvia, Chong Kun Dang and MSD Korea have not reached agreement during negotiation for nearly two years. While the distribution industry continues to demand compensation for losses incurred due to the drug price cut, the lac
- Company
- Daiichi Sankyo and Ono Pharmaceutical see surge in sales
- by Son, Hyung Min Jul 14, 2025 06:03am
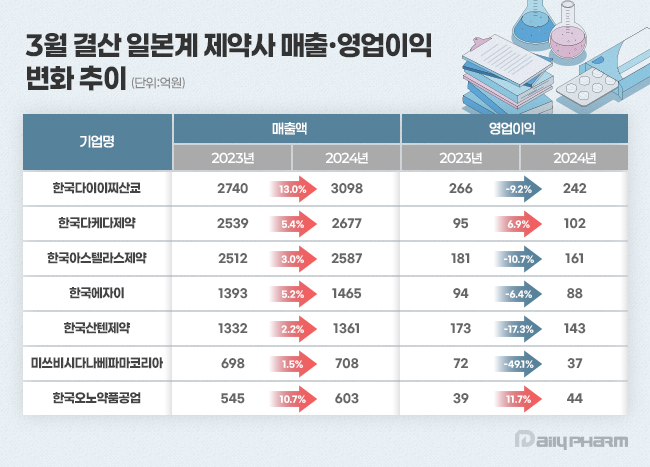
- Japanese pharmaceutical companies operating in South Korea saw overall sales growth last year. All seven companies surveyed achieved year-on-year sales growth, with Daiichi Sankyo Korea and Ono Pharma Korea leading the way with double-digit growth rates. However, in terms of operating profits, the companies showed mixed emotions. According to
- Company
- Bimzelx may be prescribed at general hospitals in KOR
- by Eo, Yun-Ho Jul 11, 2025 06:12am
- The new psoriasis drug Bimzelx may be prescribed at general hospitals in Korea. According to industry sources, UCB Pharma Korea's Bimzelx (bimekizumab) has been approved by the Drug Committees (DCs) of major hospitals nationwide, including tertiary hospitals like Seoul Asan Medical Center and Severance Hospital as well as major hospitals
- Company
- Gastric cancer-targeted therapy 'Vyloy' seeks reimb again
- by Eo, Yun-Ho Jul 10, 2025 06:10am
- Vyloy, a gastric cancer-targeted therapy, is once again vying for inclusion in the National Health Insurance reimbursement list. According to industry sources, Astellas Pharma Korea recently submitted a reimbursement application for Vyloy (zolbetuximab), a targeted treatment for Claudin 18.2-positive gastric cancer. Vyloy did not pass
- Company
- Ono Pharma Korea’s sales double in 4 years with Opdivo
- by Son, Hyung Min Jul 10, 2025 06:09am
- Ono Pharmaceutical Korea continued its sales growth, powered by its immuno-oncology drug Opdivo. The company's sales doubled in 4 years. The company is preparing for the expiration of Opdivo's patent by securing a diverse pipeline of new anticancer drugs, including antibody-drug conjugates (ADCs) and novel immuno-oncology drugs with different me
- Company
- Credit ratings for Samsung Biologics·JW Holdings↑
- by Kim, Jin-Gu Jul 10, 2025 06:08am
- Major pharmaceutical and biotech companies (biopharma companies) are facing varying differing credit ratings and outlooks. While Samsung Biologics and JW Holdings received upward credit ratings, Handok was downgraded. Dong-A ST's credit rating outlook shifted from 'stable' to 'negative'. Credit rating agencies explained that these resul
- Company
- 'Ebglyss' can be prescribed at general hospitals
- by Eo, Yun-Ho Jul 9, 2025 06:10am
- 'Ebglyss,' a new drug for the treatment of atopic dermatitis, is now available for prescription at general hospitals. According to industry sources, Lily Korea's interleukin (IL)-13 inhibitor 'Ebglyss (lebrikizumab)' has passed the drug committees (DC) of tertiary general hospitals, including Seoul National University Hospital, Asan Medic
- Company
- Adding amiloride effective in resistant hypertension
- by Son, Hyung Min Jul 9, 2025 06:08am
- A new treatment option has been proposed for patients with resistant hypertension that cannot be controlled even with the existing triple combination therapy for hypertension. A regimen combining an olmesartan-based triple combination therapy with the potassium-sparing diuretic ‘amiloride’ demonstrated similar blood pressure-lowering effec
- Company
- Novo Nordisk's injectables are in short supply
- by Nho, Byung Chul Jul 8, 2025 06:35am
- Novo Nordisk's diabetes insulin injections are experiencing a short-term shortage, causing supply difficulties in prescribing settings in Korea. The products with limited supply include NovoRapid Flexpen·Novomix Flexpen·Levemir Flexpen. Novo Nordisk sent a notice to distributors, pharmacies, hospitals, and clinics late last month, inf
- Company
- PCV21 emerges…evidence-based vaccination policies discussed
- by Whang, byung-woo Jul 7, 2025 06:10am
- "To improve pneumococcal disease prevention in Korea, an evidence-based pneumococcal vaccination policy is essential. It's crucial to evaluate the efficacy of existing vaccines and conduct cost-effectiveness assessments for new vaccines based on domestic data." Despite the implementation of the National Immunization Program (NIP), pneumococca