- Company
- Will JAK inhibitors for inflammation expand mkt presence?
- by Moon, sung-ho May 28, 2025 05:54am
- It has been confirmed that Janus kinase (JAK) inhibitors are more effective at rapidly and powerfully controlling inflammatory responses in atopic dermatitis compared to biologics. Now that switching therapies between different drug classes is allowed, this finding is expected to serve as a key basis for drug selection in clinical practice.
- Company
- Will Imfinzi finally be reimbursed for biliary tract cancer?
- by Eo, Yun-Ho May 27, 2025 06:18am
- With the advent of an era in which a single drug is used for multiple indications, awareness is growing on the need to address the issue of non-reimbursed indications. In particular, in order to improve Korea’s rigid reimbursement evaluation system, which is regarded as the main cause of reimbursement delays, not only using the flexible
- Company
- K-Bios head to ASCO…anticancer drugs to AI predictions
- by Kim, Jin-Gu May 27, 2025 06:18am
- Korean pharmaceutical and biotech companies set out to participate in the 2025 American Society of Clinical Oncology (ASCO) Annual Meeting, which is just 5 days away. At ASCO 2025, LG Chem's U.S. subsidiary Aveo Oncology, along with Tium Bio, Onconic Therapeutics, and ImmuneOncia, will each present clinical trial results about their anti
- Company
- 'Wegovy' dominating the South Korean obesity drug market
- by Chon, Seung-Hyun May 27, 2025 06:17am
- Novo Nordisk's Wegovy has dominated the South Korean obesity treatment market, establishing a monopolistic competition with over 70% market share. In just six months since its launch in Korea, Wegovy generated a sensation, surpassing KRW 100 billion in cumulative sales. Wegovy's success has expanded the obesity drug market to its largest size ev
- Company
- Re-evaluation possibility of 'Bylvay' gathers attention
- by Eo, Yun-Ho May 26, 2025 05:57am
- Attention has been drawn to when 'Bylvay Cap,' the first medicine chosen for the 'Pilot Project for Integration of Product Approvals, Reimbursement Coverage Reviews, and Drug Price Negotiations,' will be re-evaluated. Bylvay (odevixibat), Ipsen Korea's treatment option for progressive familial intrahepatic cholestasis (PFIC) in patients a
- Company
- Pfizer’s Lorviqua is granted reimbursement in Korea
- by Whang, byung-woo May 26, 2025 05:54am
- Lorviqua (lorlatinib), a treatment for ALK-mutated non-small cell lung cancer (NSCLC), has been approved for reimbursement as a first-line treatment, heralding a tectonic shift in the field. Experts saw this as a positive development in addressing unmet patient needs and improving access to treatment. In this sense, the presence of third-gene
- Company
- Accomplishments of the approval-drug price pilot project
- by Eo, Yun-Ho May 23, 2025 05:52am
- With the drugs that were reviewed for approval and drug prices simultaneously about to be commercialized, the industry gathers attention. The Ministry of Health and Welfare (MOHW) has been running the 'Pilot Project for Integration of Product Approvals, Reimbursement Coverage Reviews, and Drug Price Negotiations' since 2023 to improve tre
- Company
- CKD-Novartis new drug advances to the next phase trial
- by Chon, Seung-Hyun May 23, 2025 05:52am
- Chong Kun Dang (CKD)'s new drug candidate out-licensed to Novartis is entering the next clinical stage. CKD has secured its first milestone payment of KRW 7 billion, 1 year and 6 months after the technology export contract. CKD announced on May 22 that it expects to receive a milestone payment of USD 5 million (KRW 7 billion) from Novartis
- Company
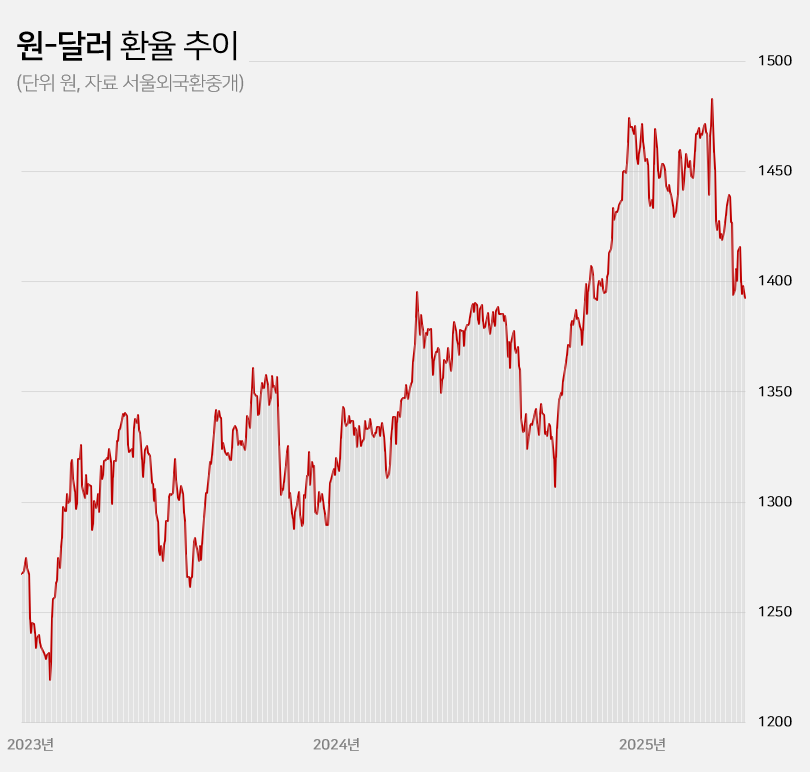
- Won-dollar rate lowest in 6mths... industry mixed
- by Kim, Jin-Gu May 23, 2025 05:51am
- With the won-dollar exchange rate falling below KRW 1,400, pharmaceutical and biotech companies are experiencing a mix of anticipation and concern. If the prolonged high exchange rate returns to previous levels, API imports and overseas clinical trial costs are expected to decrease, leading to an improvement in the cost structure. On the othe
- Company
- Will Tibsovo be reimbursed for cholangiocarcinoma this time?
- by Eo, Yun-Ho May 22, 2025 06:10am
- Attention is focused on whether the targeted anticancer drug “Tibsovo” for cholangiocarcinoma and acute myeloid leukemia will be successful in its attempt to be reimbursed by health insurance in Korea. Servier&160;Korea’s IDH1 (isocitrate dehydrogenase 1) genetic mutation targeting therapy recently passed the Health Insurance Review an