- Company
- ‘Wegovy, a game-changer for high-risk obesity patients’
- by Whang, byung-woo Jun 26, 2025 06:08am
- Obesity is a cause of various metabolic syndromes and a major risk factor for cardiovascular disease. In fact, approximately 80% of patients hospitalized for cardiovascular disease are obese, and studies have shown that the risk of cardiovascular events in obese patients is up to twice as high as in those of normal weight. Over the past 20 ye
- Company
- The 2nd KRAS-targeted cancer drug 'Krazati' expected
- by Eo, Yun-Ho Jun 26, 2025 06:08am
- The second KRAS inhibitor is expected to be commercialized in South Korea. Bristol Myers Squibb (BMS) Korea recently submitted a marketing authorization application to the Ministry of Food and Drug Safety (MFDS) for its anti-cancer drug, Krazati (adagrasib). Krazati was also designated as an orphan drug in January. It is indicated for
- Company
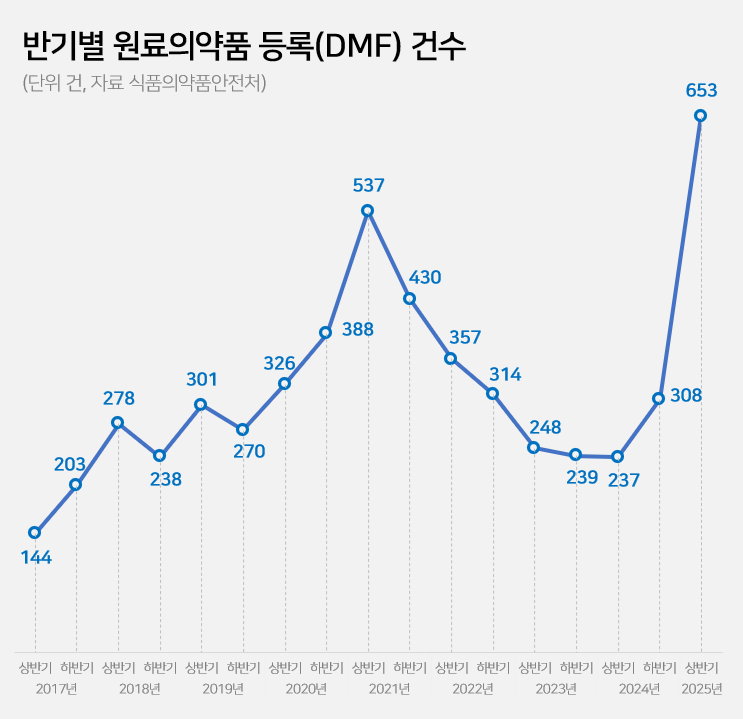
- Drug Master File for API 237→653…'easing of regulation'
- by Kim, Jin-Gu Jun 26, 2025 06:07am
- The number of Drug Master File, DMF, cases in the first half of this year surged by 2.8 times compared to the same period last year. This is the highest for a half-year period. Analysis suggests that this is due to the easing of Active Pharmaceutical Ingredient (API) registration requirements at the beginning of the year. The government had
- Company
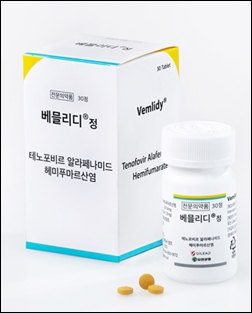
- Vemlidy indication extended to children aged 6 and older
- by Whang, byung-woo Jun 25, 2025 06:01am
- Gilead Sciences Korea announced on the 24th that its hepatitis B treatment Vemlidy (tenofovir alafenamide, TAF) has been approved by the Ministry of Food and Drug Safety for the treatment of chronic hepatitis B in children aged 6 years and older. Vemlidy offers improved renal and bone safety compared to existing chronic hepatitis B treatm
- Company
- K-Bio successfully signs multiple technology transfer deals
- by Son, Hyung Min Jun 25, 2025 06:01am
- In the first half of this year, the Korean pharmaceutical and biotech industry has successfully achieved technology transfers in various areas, including changed formulations for immunotherapy, new obesity drug candidates, and new drug discovery platforms. Companies, such as LigaChem Biosciences and Onconic Therapeutics, have successfully receiv
- Company
- CSL Seqirus partners with Samjin...seeks NIP in Korea
- by Whang, byung-woo Jun 24, 2025 06:02am
- CSL Seqirus Korea and Samjin Pharmaceutical have signed a strategic sales partnership agreement for the domestic distribution of influenza vaccines, sparking attention over whether the two companies can achieve synergistic effects. On the 18th, the two companies announced that they had signed a strategic sales partnership agreement for the
- Company
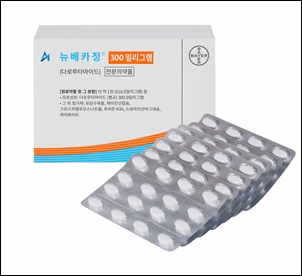
- Nubeqa’s indication expanded to msHPC in combo with ADT
- by Whang, byung-woo Jun 24, 2025 06:00am
- Bayer Korea announced on the 23rd that its oral androgen receptor inhibitor (ARi) Nubeqa (darolutamide) has been approved by the Ministry of Food and Drug Safety as part of a two-drug regimen in combination with androgen deprivation therapy (ADT) for the treatment of metastatic hormone-sensitive prostate cancer (mHSPC). With this expanded
- Company
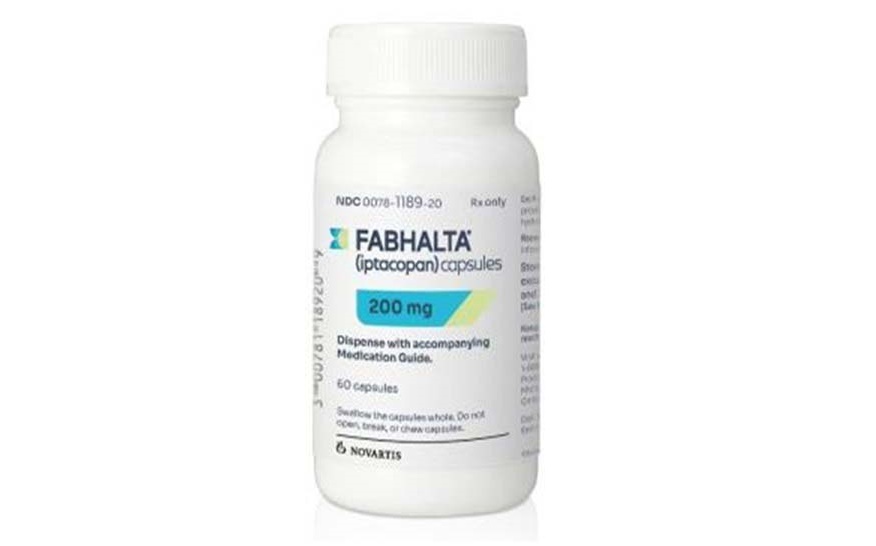
- 'Fabhalta' closer to being reimbursed, shaking up inj market
- by Moon, sung-ho Jun 24, 2025 06:00am
- Next month, new oral agent will be introduced to the market for paroxysmal nocturnal hemoglobinuria (PNH). Injectables, such as Soliris and Ultomiris, currently dominate the PNH market. Attention is drawn to whether this oral agent will enhance patient satisfaction, as it offers the convenience of administration compared to the previous treat
- Company
- Tibsovo may be prescribed at general hospitals in KOR
- by Eo, Yun-Ho Jun 24, 2025 05:59am
- Tibsovo, a targeted cancer drug for cholangiocarcinoma and acute myeloid leukemia, can now be prescribed at general hospitals in Korea. According to industry sources, Servier&160;Korea’s IDH1 (isocitrate dehydrogenase 1) genetic mutation targeting therapy Tibsovo (ivosidenib) recently passed the Drug Committee (DC) review of the ‘Big 5
- Company
- Eylea biosimilar pharmas receive differing injunction ruling
- by Kim, Jin-Gu Jun 23, 2025 06:01am
- Domestic biosimilar companies have launched a full-scale patent lawsuit against Bayer over Eylea. Celltrion and Samsung Bioepis received different preliminary injunction results, leading them to pursue divergent strategies in the main lawsuit. Eylea patent infringement main lawsuits begin in earnest... Celltrion to proceed immediately, Sam